Dental Sterilization Summary
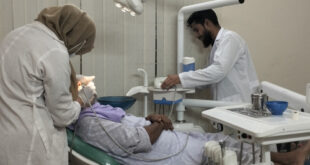
Dental Sterilization. Mobile Phone 01797522136, 01987073965. Dental Sterilization is a process that removes, kills, or deactivates all kinds of microorganisms such as bacteria, viruses, fungi, and protozoa. This process is important for all kinds of surgeries.
দাঁতের জীবাণুমুক্তকরণ। মোবাইল ফোন 01797522136, 01987073965। দাঁতের জীবাণুমুক্তকরণ হল এমন একটি প্রক্রিয়া যা ব্যাকটেরিয়া, ভাইরাস, ছত্রাক এবং প্রোটোজোয়ার মতো সব ধরনের অণুজীবকে অপসারণ করে, মেরে ফেলে বা নিষ্ক্রিয় করে। এই প্রক্রিয়াটি সব ধরনের সার্জারির জন্য গুরুত্বপূর্ণ।

Dental Instruments are reusable. So, Dental Instruments should be sterilized after surgical use of it. Dental Sterilization is a practical work. All Dental Courses provide these practicals. Dental Courses are a Dental Training Course, a Diploma in Dental Assistant, a Diploma in Dental Technology, and a Diploma in Dental. All Courses are available at HRTD Medical Institute.
ডেন্টাল ইন্সট্রুমেন্ট পুনরায় ব্যবহারযোগ্য। সুতরাং, ডেন্টাল ইন্সট্রুমেন্টগুলি অস্ত্রোপচারের পরে এটিকে জীবাণুমুক্ত করা উচিত। দাঁতের জীবাণুমুক্তকরণ একটি ব্যবহারিক কাজ। সমস্ত ডেন্টাল কোর্স এই ব্যবহারিক প্রদান করে। ডেন্টাল কোর্স হল ডেন্টাল ট্রেনিং কোর্স, ডেন্টাল অ্যাসিস্ট্যান্টে ডিপ্লোমা, ডেন্টাল টেকনোলজিতে ডিপ্লোমা এবং ডেন্টালে ডিপ্লোমা। HRTD মেডিকেল ইনস্টিটিউটে সমস্ত কোর্স পাওয়া যায়।
1. Define Sterilization.
Sterilization is a process that removes, kills, or deactivates all kinds of microorganisms such as bacteria, viruses, fungi, and protozoa. This process is very important for all kinds of surgery.
জীবাণুমুক্তকরণ এমন একটি প্রক্রিয়া যা ব্যাকটেরিয়া, ভাইরাস, ছত্রাক এবং প্রোটোজোয়ার মতো সমস্ত ধরণের অণুজীবকে অপসারণ করে, মেরে ফেলে বা নিষ্ক্রিয় করে। এই প্রক্রিয়াটি সব ধরনের সার্জারির জন্য খুবই গুরুত্বপূর্ণ।
2. What are the 3 types of sterilization?
Three primary methods of medical sterilization occur from high temperature/pressure and chemical processes.
উচ্চ তাপমাত্রা/চাপ এবং রাসায়নিক প্রক্রিয়া থেকে চিকিৎসা নির্বীজন করার তিনটি প্রাথমিক পদ্ধতি ঘটে।
- Plasma Gas Sterilization (By Plasma Gas Sterilizers)
- Autoclave Sterilization ( Sterilization by Autoclaves)
- Vaporized Hydrogen Peroxide Sterilization (Sterilization by Vaporized Hydrogen Peroxide Sterilizers)
3. Define Dental Sterilization.
Sterilization is an essential step in reprocessing reusable dental instruments that have become contaminated or are potentially contaminated with saliva blood or other biological fluids. This includes dental handpieces.
জীবাণুমুক্তকরণ হল পুনঃব্যবহারযোগ্য দাঁতের যন্ত্রপাতি যেগুলি দূষিত হয়ে গেছে বা লালা রক্ত বা অন্যান্য জৈবিক তরল দ্বারা সম্ভাব্য দূষিত হয়েছে পুনঃপ্রক্রিয়াকরণের একটি অপরিহার্য পদক্ষেপ। এর মধ্যে রয়েছে ডেন্টাল হ্যান্ডপিস।
4. Why Dental Sterilization is important?
Importance of Dental Sterilization (দাঁতের জীবাণুমুক্তকরণের গুরুত্ব) :
Dental equipment sterilization protects dentists and patients. Sound dental sterilization practices in dentistry protect patients dentists and the whole team. They prevent the growth of bacteria on instruments and surfaces throughout the dental practice.
ডেন্টাল সরঞ্জাম নির্বীজন ডেন্টিস্ট এবং রোগীদের রক্ষা করে। দন্তচিকিৎসায় সাউন্ড ডেন্টাল নির্বীজন অনুশীলন রোগীদের ডেন্টিস্ট এবং পুরো দলকে রক্ষা করে। তারা দাঁতের অনুশীলন জুড়ে যন্ত্র এবং পৃষ্ঠগুলিতে ব্যাকটেরিয়া বৃদ্ধি রোধ করে।
Sterilization of Dental Instruments
1. Introduction-
Decontamination Into Practice is part of a national initiative to promote and standardize good decontamination practice in dental primary care.
Part 1: Cleaning of Dental Instrument(Published in 2007) deals with how to clean dental instruments thoroughly, including thermal disinfection using a washer disinfector, and advice about rinsing drying, and inspection of the instruments after cleaning.
পার্ট 1: ডেন্টাল ইন্সট্রুমেন্টের ক্লিনিং অফ ডেন্টাল ইন্সট্রুমেন্ট (2007 সালে প্রকাশিত) একটি ওয়াশার ডিসইনফেক্টর ব্যবহার করে থার্মাল ডিসইনফেকশন সহ ডেন্টাল ইন্সট্রুমেন্টগুলিকে কীভাবে ভালভাবে পরিষ্কার করা যায়, এবং শুষ্ক করার পরামর্শ এবং পরিষ্কারের পরে যন্ত্রগুলি পরিদর্শন করার বিষয়ে পরামর্শ দেয়।
Part 2: Sterilization of Dental Instruments focuses on how to sterilize dental instruments after cleaning, using small steam sterilizers. It provides advice that is based on health and safety regulations and current technical guidance on sterilization within healthcare. It has been developed through consultation with various experts and end users.
পার্ট 2: ডেন্টাল ইন্সট্রুমেন্টের জীবাণুমুক্তকরণ ছোট বাষ্প নির্বীজনকারী ব্যবহার করে পরিষ্কারের পরে দাঁতের যন্ত্রগুলিকে কীভাবে জীবাণুমুক্ত করা যায় তার উপর ফোকাস করে। এটি পরামর্শ প্রদান করে যা স্বাস্থ্য ও নিরাপত্তা প্রবিধানের উপর ভিত্তি করে এবং স্বাস্থ্যসেবার মধ্যে নির্বীজন সংক্রান্ত বর্তমান প্রযুক্তিগত নির্দেশিকা। এটি বিভিন্ন বিশেষজ্ঞ এবং শেষ ব্যবহারকারীদের সাথে পরামর্শের মাধ্যমে তৈরি করা হয়েছে।
The advice in this document aims to be practical and achievable with the equipment most commonly used in the primary care dental practice environment. There are risks associated with the reuse of instruments. By adopting procedures consistent with this guidance in dental practices in Scotland, a very significant risk reduction and an improvement in decontamination and patient safety will be achieved. As new knowledge and technology develop it may be necessary to update this guidance.
এই নথির উপদেশটি প্রাথমিক যত্নের দাঁতের অনুশীলন পরিবেশে সাধারণত ব্যবহৃত সরঞ্জামগুলির সাথে ব্যবহারিক এবং অর্জনযোগ্য হওয়ার লক্ষ্য। যন্ত্রের পুনঃব্যবহারের সাথে যুক্ত ঝুঁকি আছে। স্কটল্যান্ডে দাঁতের অনুশীলনে এই নির্দেশনার সাথে সামঞ্জস্যপূর্ণ পদ্ধতিগুলি গ্রহণ করার মাধ্যমে, একটি অত্যন্ত গুরুত্বপূর্ণ ঝুঁকি হ্রাস এবং দূষণমুক্তকরণ এবং রোগীর নিরাপত্তার উন্নতি সাধিত হবে। নতুন জ্ঞান এবং প্রযুক্তির বিকাশের সাথে সাথে এই নির্দেশিকা আপডেট করার প্রয়োজন হতে পারে।
Supplementary information is provided in the introduction and appendices of the Decontamination Into Practice series. Many of the web links included can be accessed via the Decontamination section of the Scottish Dental website: www.scottishdental.org. Notably, the following Scottish Health Technical Memoranda (SHTM) has detailed information on how to choose, use, and validate equipment for decontamination processes.
পরিপূরক তথ্য ডিকনটামিনেশন ইনটু প্র্যাকটিস সিরিজের ভূমিকা এবং পরিশিষ্টে প্রদান করা হয়েছে। অন্তর্ভুক্ত অনেক ওয়েব লিঙ্ক স্কটিশ ডেন্টাল ওয়েবসাইট: www.scottishdental.org-এর ডিকনটামিনেশন বিভাগের মাধ্যমে অ্যাক্সেস করা যেতে পারে। উল্লেখযোগ্যভাবে, নিম্নোক্ত স্কটিশ হেলথ টেকনিক্যাল মেমোরান্ডা (SHTM)-এ কীভাবে দূষণমুক্তকরণ প্রক্রিয়ার জন্য সরঞ্জাম নির্বাচন, ব্যবহার এবং যাচাই করা যায় সে সম্পর্কে বিস্তারিত তথ্য রয়েছে।
- SHTM 2010 Sterilization
- SHTM 2030 Washer-disinfectors (includes ultrasonic cleaners )
As Sterilization is a highly technical activity, on occasion it may be necessary to consult an Authorising Engineer (Decontamination ) for specific advice concerning validation, periodic testing, maintenance, and operational management as defined in SHTM 2010. The Authorising Engineer (Decontamination) service for NHS Scotland is provided by Health Facilities Scotland (see Appendix 5 ). Note that at the time of writing, there are relatively few of these specialists to advise both secondary and primary care services.
যেহেতু জীবাণুমুক্তকরণ একটি অত্যন্ত প্রযুক্তিগত ক্রিয়াকলাপ, সেহেতু SHTM 2010-এ সংজ্ঞায়িত বৈধকরণ, পর্যায়ক্রমিক পরীক্ষা, রক্ষণাবেক্ষণ এবং অপারেশনাল ম্যানেজমেন্ট সম্পর্কিত নির্দিষ্ট পরামর্শের জন্য কখনও কখনও অনুমোদন প্রকৌশলী (ডিকনটামিনেশন) এর সাথে পরামর্শ করার প্রয়োজন হতে পারে। এনএইচএস স্কটল্যান্ড স্বাস্থ্য সুবিধা স্কটল্যান্ড দ্বারা সরবরাহ করা হয়েছে (পরিশিষ্ট 5 দেখুন)। উল্লেখ্য যে লেখার সময়, মাধ্যমিক এবং প্রাথমিক উভয় পরিচর্যা পরিষেবার পরামর্শ দেওয়ার জন্য এই বিশেষজ্ঞদের মধ্যে তুলনামূলকভাবে খুব কমই আছেন।
Sterilization in the Dental Practice-
The decontamination of reusable dental instruments includes:
- cleaning (পরিষ্কার করা)
- rinsing ( ধৌত করা )
- drying (শুকানো)
- inspection for dryness, functionality, and cleanliness (শুষ্কতা, কার্যকারিতা এবং পরিচ্ছন্নতার জন্য পরিদর্শন)
- wrapping before sterilization when using a vacuum sterilizer
(ভ্যাকুয়াম নির্বীজনকারী ব্যবহার করার সময় নির্বীজন করার আগে মোড়ানো)
- sterilization (জীবাণুমুক্তকরণ)
- wrapping after sterilization when using a non-vacuum sterilizer (একটি নন-ভ্যাকুয়াম নির্বীজনকারী ব্যবহার করার সময় নির্বীজন করার পরে মোড়ানো)
Sterilization is an essential step in the reprocessing of reusable dental instruments that have become contaminated or are potentially contaminated with saliva, blood, or other biological fluids. This includes dental handpieces. The aim of sterilization is to break the chain of potential cross-infection between patients by killing microorganisms, including spores. However, prion proteins are not fully deactivated by the sterilization process. Therefore effective instrument cleaning is particularly important to physically remove contamination, including prion proteins, prior to sterilization.
জীবাণুমুক্তকরণ হল পুনঃব্যবহারযোগ্য দাঁতের যন্ত্রগুলির পুনঃপ্রক্রিয়াকরণের একটি অপরিহার্য পদক্ষেপ যা দূষিত হয়ে গেছে বা লালা, রক্ত বা অন্যান্য জৈবিক তরল দ্বারা সম্ভাব্য দূষিত। এর মধ্যে রয়েছে ডেন্টাল হ্যান্ডপিস। জীবাণুমুক্তকরণের লক্ষ্য হ'ল স্পোর সহ অণুজীবগুলিকে হত্যা করে রোগীদের মধ্যে সম্ভাব্য ক্রস-সংক্রমণের শৃঙ্খলটি ভেঙে দেওয়া। যাইহোক, প্রান প্রোটিনগুলি নির্বীজন প্রক্রিয়া দ্বারা সম্পূর্ণরূপে নিষ্ক্রিয় হয় না। তাই জীবাণুমুক্ত করার আগে শারীরিকভাবে দূষণ দূর করার জন্য কার্যকরী যন্ত্র পরিষ্কার করা বিশেষভাবে গুরুত্বপূর্ণ।
Sterilization using a steam sterilizer is recommended as the most efficient, cost-effective, and safe method of sterilizing dental instruments in primary care dental practices. The sterilization process must be validated to ensure that instruments are reliably and consistently sterilized using predetermined and reproducible conditions.
প্রাথমিক যত্নের দাঁতের অনুশীলনে দাঁতের যন্ত্রপাতি জীবাণুমুক্ত করার সবচেয়ে কার্যকর, সাশ্রয়ী এবং নিরাপদ পদ্ধতি হিসাবে বাষ্প নির্বীজনকারী ব্যবহার করে জীবাণুমুক্ত করার সুপারিশ করা হয়। পূর্বনির্ধারিত এবং পুনরুৎপাদনযোগ্য শর্তগুলি ব্যবহার করে যন্ত্রগুলি নির্ভরযোগ্যভাবে এবং ধারাবাহিকভাবে জীবাণুমুক্ত হয় তা নিশ্চিত করার জন্য নির্বীজন প্রক্রিয়াটি অবশ্যই যাচাই করা উচিত।
To kill microorganisms, the instruments need to be exposed to steam at a specified temperature for a specific holding time. Although other options exist, the preferred temperature-pressure-time relationship for all small steam sterilizers is 134-137C, 2.1-2.25 bar gauge pressure for at least a 3-minute holding time.
অণুজীবকে মেরে ফেলার জন্য, যন্ত্রগুলিকে একটি নির্দিষ্ট তাপমাত্রায় একটি নির্দিষ্ট ধরে রাখার সময়ের জন্য বাষ্পের সংস্পর্শে আসতে হবে। যদিও অন্যান্য বিকল্পগুলি বিদ্যমান, সমস্ত ছোট বাষ্প নির্বীজনকারীর জন্য পছন্দের তাপমাত্রা-চাপ-সময় সম্পর্ক হল 134-137C, 2.1-2.25 বার গেজ চাপ কমপক্ষে 3-মিনিট ধরে রাখার জন্য।
It is preferable to use reusable instruments that can withstand both an automated cleaning/disinfection process and steam sterilization or to use single-use instruments. Reusable instruments that cannot withstand steam sterilization must be decontaminated as recommended by the instrument manufacturer.
পুনঃব্যবহারযোগ্য যন্ত্রগুলি ব্যবহার করা বাঞ্ছনীয় যা একটি স্বয়ংক্রিয় পরিষ্কার/জীবাণুমুক্তকরণ প্রক্রিয়া এবং বাষ্প নির্বীজন উভয়ই সহ্য করতে পারে বা একক ব্যবহারের যন্ত্র ব্যবহার করতে পারে৷ পুনঃব্যবহারযোগ্য যন্ত্রগুলি যা বাষ্প নির্বীজন সহ্য করতে পারে না সেগুলিকে যন্ত্র প্রস্তুতকারকের সুপারিশ অনুসারে দূষিত করা আবশ্যক৷
Sterilization cycles in small steam sterilizers-
The sterilization cycle in a small steam sterilizer is a pre-programmed sequence of operating stages. There are three types of sterilization cycles, Type N, Type B, and Type S. These cycles differ in the manner in which air is removed, the types of load they can sterilize, and whether or not items can be
একটি ছোট স্টিম স্টেরিলাইজারে নির্বীজন চক্র হল অপারেটিং পর্যায়ের একটি পূর্ব-প্রোগ্রাম করা ক্রম। তিন ধরনের জীবাণুমুক্তকরণ চক্র রয়েছে, টাইপ এন, টাইপ বি এবং টাইপ এস। এই চক্রগুলি যেভাবে বায়ু অপসারণ করা হয়, কী ধরনের লোড তারা জীবাণুমুক্ত করতে পারে এবং আইটেমগুলি হতে পারে কি না তার মধ্যে পার্থক্য রয়েছে।
As some sterilizers can perform more than one type of sterilization cycle, it is more correct to refer to the type of cycle performed rather than the type of machine. However, the following terms are often used for convenience.
যেহেতু কিছু জীবাণু নির্বীজনকারী একাধিক ধরণের নির্বীজন চক্র সম্পাদন করতে পারে, তাই মেশিনের প্রকারের পরিবর্তে সঞ্চালিত চক্রের ধরণ উল্লেখ করা আরও সঠিক। যাইহোক, নিম্নলিখিত পদগুলি প্রায়ই সুবিধার জন্য ব্যবহৃত হয়।
- Non-vacuum sterilizer or type N sterilizer
- Vacuum sterilizer or type B sterilizer
This guidance describes the sterilization of unwrapped instruments in any type of sterilizer and wrapped instruments in a vacuum sterilizer, but not specifically a type S sterilizer. This is because the various makes of type S sterilizers differ in the type of load they can be used for and some may not be suitable for sterilizing wrapped instruments. Refer to the manufactures instructions for advice on the use of type S sterilizers.
এই নির্দেশিকাটি যেকোন ধরণের জীবাণুনাশক এবং ভ্যাকুয়াম জীবাণুমুক্ত যন্ত্রে মোড়ানো যন্ত্রের জীবাণুমুক্তকরণকে বর্ণনা করে, তবে বিশেষভাবে একটি টাইপ এস জীবাণুমুক্ত নয়। কারণ এস স্টেরিলাইজারের বিভিন্ন ধরনের লোডের মধ্যে পার্থক্য রয়েছে যেগুলির জন্য তারা ব্যবহার করা যেতে পারে এবং কিছু মোড়ানো যন্ত্র জীবাণুমুক্ত করার জন্য উপযুক্ত নাও হতে পারে। টাইপ এস স্টেরিলাইজার ব্যবহারের পরামর্শের জন্য প্রস্তুতকারকের নির্দেশাবলী পড়ুন।
Sterilized versus sterile
Instruments are regarded as sterilized when they
have been cleaned, and inspected when wrapped before being sterilized in a sterilizer designed to process wrapped instruments (e. g a vacuum sterilizer) to maintain sterility these instruments must be stored with the wrapping intact until immediately before use
পরিষ্কার করা হয়েছে, এবং জীবাণুমুক্ত করার আগে মোড়ানো যন্ত্রে (যেমন একটি ভ্যাকুয়াম জীবাণুনাশক) প্রক্রিয়াকরণের জন্য পরিকল্পিত জীবাণুমুক্ত করার সময় পরিদর্শন করা হয়েছে বন্ধ্যাত্ব বজায় রাখার জন্য এই যন্ত্রগুলি ব্যবহার করার অবিলম্বে আগে পর্যন্ত র্যাপিং অক্ষত অবস্থায় সংরক্ষণ করতে হবে
or,
are bought as sterile single-use items and used in accordance with manufacturer instructions. (i. e. used immediately on removal from the sterile pack and used only once)
জীবাণুমুক্ত একক ব্যবহারের আইটেম হিসাবে কেনা হয় এবং প্রস্তুতকারকের নির্দেশাবলী অনুসারে ব্যবহার করা হয়।
Sterilization of Dental Handpieces-
There is currently no agreed method for the effective decontamination of dental handpieces. Research to assess the effectiveness of various methods of handpiece decontamination is ongoing. At present, it is best practice to follow manufactures instructions for handpiece cleaning. After cleaning it is then essential to sterilize handpieces in a steam sterilizer. Although the effectiveness of sterilization of the internal structures is unclear, processing in a sterilizer ensures that the external surfaces are sterilized and may also contribute to risk reduction through further thermal disinfection of the internal structures.
দাঁতের হ্যান্ডপিস কার্যকরভাবে দূষণমুক্ত করার জন্য বর্তমানে কোনো সম্মত পদ্ধতি নেই। হ্যান্ডপিস দূষণমুক্ত করার বিভিন্ন পদ্ধতির কার্যকারিতা মূল্যায়নের জন্য গবেষণা চলছে। বর্তমানে, হ্যান্ডপিস পরিষ্কারের জন্য প্রস্তুতকারকের নির্দেশাবলী অনুসরণ করা সর্বোত্তম অনুশীলন। পরিষ্কার করার পরে এটি একটি বাষ্প জীবাণুমুক্ত করার হাতের টুকরা জীবাণুমুক্ত করা অপরিহার্য। যদিও অভ্যন্তরীণ কাঠামোর জীবাণুমুক্তকরণের কার্যকারিতা স্পষ্ট নয়, জীবাণুমুক্তকরণে প্রক্রিয়াকরণ নিশ্চিত করে যে বাহ্যিক পৃষ্ঠগুলি জীবাণুমুক্ত করা হয়েছে এবং অভ্যন্তরীণ কাঠামোর আরও তাপীয় জীবাণুমুক্তকরণের মাধ্যমে ঝুঁকি হ্রাসে অবদান রাখতে পারে।
- When purchasing new handpieces, ensure that they can withstand thermal disinfection and steam sterilization.
- Always process dental handpieces in a steam sterilizer as part of their decontamination. Replace existing handpieces that cannot withstand steam sterilization.
- Follow the handpiece manufacturer’s decontamination instructions.
- If necessary, contact the handpiece manufacturer to request clarification of their instructions.
- Lubricate handpieces before and/or after sterilization as recommended by the manufacturer. If lubrication is required both before and after sterilization, use separate designated cleaned only and sterilized canisters of lubricant labeled accordingly.
- Automated ‘handpiece cleaning machines’ can be used to lubricate handpieces. These machines are not validated for cleaning and do not disinfect. However, their use may prolong handpiece life and can be particularly useful if handpieces are cleaned in a washer disinfector. See also cleaning of dental instruments for advice on alternative methods for cleaning handpieces and care of handpieces after cleaning.
নতুন হ্যান্ডপিস কেনার সময়, নিশ্চিত করুন যে তারা তাপ নির্বীজন এবং বাষ্প নির্বীজন সহ্য করতে পারে। দাঁতের হ্যান্ডপিসগুলিকে দূষণমুক্ত করার অংশ হিসাবে সর্বদা একটি স্টিম স্টেরিলাইজারে প্রক্রিয়া করুন। বিদ্যমান হ্যান্ডপিসগুলি প্রতিস্থাপন করুন যা বাষ্প নির্বীজন সহ্য করতে পারে না। হ্যান্ডপিস প্রস্তুতকারকের দূষণমুক্ত করার নির্দেশাবলী অনুসরণ করুন। প্রয়োজনে, হ্যান্ডপিস প্রস্তুতকারকের সাথে যোগাযোগ করুন তাদের নির্দেশাবলীর ব্যাখ্যার জন্য অনুরোধ করুন। প্রস্তুতকারকের সুপারিশ অনুযায়ী জীবাণুমুক্ত করার আগে এবং/বা পরে হ্যান্ডপিসগুলিকে লুব্রিকেট করুন। যদি জীবাণুমুক্তকরণের আগে এবং পরে উভয় ক্ষেত্রেই তৈলাক্তকরণের প্রয়োজন হয়, তবে পৃথক মনোনীত পরিষ্কার করা এবং সেই অনুযায়ী লেবেলযুক্ত লুব্রিকেন্টের জীবাণুমুক্ত ক্যানিস্টার ব্যবহার করুন। স্বয়ংক্রিয় 'হ্যান্ডপিস ক্লিনিং মেশিন' হ্যান্ডপিস লুব্রিকেট করতে ব্যবহার করা যেতে পারে। এই মেশিনগুলি পরিষ্কারের জন্য বৈধ নয় এবং জীবাণুমুক্ত করে না। যাইহোক, তাদের ব্যবহার হ্যান্ডপিসের জীবনকে দীর্ঘায়িত করতে পারে এবং বিশেষভাবে কার্যকর হতে পারে যদি হাতের টুকরাগুলিকে একটি ওয়াশার জীবাণুনাশক দিয়ে পরিষ্কার করা হয়। এছাড়াও হ্যান্ডপিস পরিষ্কার করার বিকল্প পদ্ধতি এবং পরিষ্কারের পরে হ্যান্ডপিসগুলির যত্নের পরামর্শের জন্য দাঁতের যন্ত্র পরিষ্কার করা দেখুন।
- 2. Organising Sterilization Within the Decontamination Area-,./;
Purchasing a small steam sterilizer-
Before purchasing a small steam sterilizer, to ensure that it is suitable for your sure:
একটি ছোট বাষ্প নির্বীজনকারী কেনার আগে, এটি আপনার নিশ্চিত করার জন্য উপযুক্ত কিনা তা নিশ্চিত করতে:
Specify clearly to the supplier the Type of loads that you intend to reprocess including:
আপনি যে ধরনের লোডগুলি পুনঃপ্রক্রিয়া করতে চান তা সরবরাহকারীর কাছে স্পষ্টভাবে উল্লেখ করুন:
- The quantities of instruments you are likely to reprocess per load and per day.
- প্রতি লোড এবং প্রতি দিনে আপনি যে পরিমাণ যন্ত্রগুলি পুনরায় প্রক্রিয়া করতে পারেন।
Ensure the sterilizer carries the CE mark. This indicates that the manufacturer claims compliance with the Essential requirements of the Medical Device Directive.
নিশ্চিত করুন যে জীবাণুনাশকটি সিই চিহ্ন বহন করে। এটি ইঙ্গিত করে যে প্রস্তুতকারক মেডিকেল ডিভাইস নির্দেশের অপরিহার্য প্রয়োজনীয়তাগুলির সাথে সম্মতি দাবি করেছেন।
Ensure that the sterilizer complies with British Standards (BS EN 13060) And SHTM 2010.
নির্বীজনকারী ব্রিটিশ স্ট্যান্ডার্ড (BS EN 13060) এবং SHTM 2010 মেনে চলছে তা নিশ্চিত করুন।
Check with the supplier that-
- they can install the sterilizer to be consistent with SHTM 2010 requirements and provide certification of this.
- তারা SHTM 2010 এর প্রয়োজনীয়তার সাথে সামঞ্জস্যপূর্ণ হওয়ার জন্য জীবাণুমুক্তকারী ইনস্টল করতে পারে এবং এটির শংসাপত্র প্রদান করতে পারে।
- they will provide written operating instructions nd training.
- তারা লিখিত অপারেটিং নির্দেশাবলী এবং প্রশিক্ষণ প্রদান করবে।
- they can guarantee an efficient repair service and response time can provide replacement equipment if necessary.
- তারা একটি দক্ষ মেরামত পরিষেবার গ্যারান্টি দিতে পারে এবং প্রতিক্রিয়া সময় প্রয়োজন হলে প্রতিস্থাপন সরঞ্জাম সরবরাহ করতে পারে।
- they can supply a contract for maintenance and testing in accordance with the manufactures instruction.
- তারা প্রস্তুতকারকের নির্দেশ অনুসারে রক্ষণাবেক্ষণ এবং পরীক্ষার জন্য একটি চুক্তি সরবরাহ করতে পারে।
- The Sterilizer performs a cycle that can be validated (see section 6).
- নির্বীজনকারী একটি চক্র সম্পাদন করে যা যাচাই করা যেতে পারে (বিভাগ 6 দেখুন)।
Ask the supplier to provide details in writing of –
সরবরাহকারীকে লিখিতভাবে বিস্তারিত জানাতে বলুন-
- how many instrument trays, cassettes or rocks the sterilizer can process in one cycle.
- জীবাণুনাশক এক চক্রে কতগুলি উপকরণ ট্রে, ক্যাসেট বা শিলা প্রক্রিয়া করতে পারে।
- how long a cycle takes.
- একটি চক্র কতক্ষণ লাগে।
- the number of different cycles the sterilizer can perform.
- জীবাণুনাশক সঞ্চালন করতে পারে বিভিন্ন চক্রের সংখ্যা।
- dimensions and door orientation.
- মাত্রা এবং দরজা অভিযোজন।
- a local servicing agent.
- একটি স্থানীয় সার্ভিসিং এজেন্ট।
- the costs involved for installation , validation, periodic testing and maintenance.
- ইনস্টলেশন, বৈধতা, পর্যায়ক্রমিক পরীক্ষা এবং রক্ষণাবেক্ষণের জন্য জড়িত খরচ।
- periodic tests, including whether the machine can perform these tests automatically or whether the user can perform them,
- পর্যায়ক্রমিক পরীক্ষা, মেশিন স্বয়ংক্রিয়ভাবে এই পরীক্ষাগুলি সম্পাদন করতে পারে কিনা বা ব্যবহারকারী সেগুলি সম্পাদন করতে পারে কিনা
- how long the machine is out of action foe maintenance and how many times per year.
- মেশিনটি কতক্ষণ রক্ষণাবেক্ষণের বাইরে থাকে এবং বছরে কতবার।
- the electrical and/or plumbing requirements.
- বৈদ্যুতিক এবং/অথবা নদীর গভীরতানির্ণয় প্রয়োজনীয়তা।
- any other specific requirements (e.g. water quality and quantity required per cycle)
- অন্য কোনো নির্দিষ্ট প্রয়োজনীয়তা (যেমন জলের গুণমান এবং সাইকেল প্রতি প্রয়োজনীয় পরিমাণ)
- whether the machine has a printer installed or an electronic data logger and if so whether this records temperature , pressure and sterilization hold time.
- মেশিনে একটি প্রিন্টার ইনস্টল করা আছে কিনা বা একটি ইলেকট্রনিক ডেটা লগার আছে কিনা এবং যদি তা হয় তবে এটি তাপমাত্রা, চাপ এবং জীবাণুমুক্ত করার সময় রেকর্ড করে কিনা।
- whether other attachments or accessories are required and whether they have been included in the costs.
- অন্যান্য সংযুক্তি বা আনুষাঙ্গিক প্রয়োজন কিনা এবং সেগুলি খরচের মধ্যে অন্তর্ভুক্ত করা হয়েছে কিনা।
The resource requirements (e.g. costs and time for testing, level of staff training) will differ significantly depending on the type of sterilizer. In 20111, following an assessment of the current literature, the Scottish Health Technologies Group determined that there is a lack of evidence to conclude that the provision of benchtop steam vacuum sterilizers in primary care dental practices in Scotland would increase patient safety and thereby justify the cost (Advice statement 003/11, which will be subject to periodic review). While cost is a concern, it is essential to follow the manufacturer’s reprocessing instructions for both sterilizer and instruments to inform your decisions about the purchase of a small steam sterilizer.
জীবাণুনাশকের প্রকারের উপর নির্ভর করে সম্পদের প্রয়োজনীয়তাগুলি (যেমন পরীক্ষার জন্য খরচ এবং সময়, কর্মীদের প্রশিক্ষণের স্তর) উল্লেখযোগ্যভাবে আলাদা হবে। 20111 সালে, বর্তমান সাহিত্যের একটি মূল্যায়নের পর, স্কটিশ হেলথ টেকনোলজিস গ্রুপ নির্ধারণ করে যে স্কটল্যান্ডে প্রাথমিক যত্নের দাঁতের অনুশীলনে বেঞ্চটপ স্টিম ভ্যাকুয়াম স্টেরিলাইজারের বিধান রোগীর নিরাপত্তা বৃদ্ধি করবে এবং এর ফলে খরচকে ন্যায্যতা দেবে ( পরামর্শ বিবৃতি 003/11, যা পর্যায়ক্রমিক পর্যালোচনা সাপেক্ষে হবে)। যদিও খরচ একটি উদ্বেগের বিষয়, একটি ছোট বাষ্প নির্বীজনকারী কেনার বিষয়ে আপনার সিদ্ধান্ত জানাতে জীবাণুনাশক এবং যন্ত্র উভয়ের জন্য প্রস্তুতকারকের পুনঃপ্রক্রিয়াকরণ নির্দেশাবলী অনুসরণ করা অপরিহার্য।
The NHSScotland National Contract for Decontamination Equipment-
এনএইচএসএসকোটল্যান্ড ন্যাশনাল কন্ট্রাক্ট ফর ডিকনট্যামিনেশন ইকুইপমেন্ট-
NHSScotland has a national contract for local decontamination unit (LDU) equipment that was created following a period of equipment testing. The contract includes the purchase price of several small steam sterilizers and gives details of the additional costs for installation, commissioning, testing, and maintenance. A full support package which includes both the equipment and the additional costs is also listed.
NHSScotland স্থানীয় ডিকনটামিনেশন ইউনিট (LDU) সরঞ্জামগুলির জন্য একটি জাতীয় চুক্তি রয়েছে যা সরঞ্জাম পরীক্ষার সময়কালের পরে তৈরি করা হয়েছিল। চুক্তিতে বেশ কয়েকটি ছোট স্টিম স্টেরিলাইজারের ক্রয় মূল্য অন্তর্ভুক্ত রয়েছে এবং ইনস্টলেশন, কমিশনিং, পরীক্ষা এবং রক্ষণাবেক্ষণের জন্য অতিরিক্ত খরচের বিবরণ দেয়। একটি সম্পূর্ণ সমর্থন প্যাকেজ যা উভয় সরঞ্জাম এবং অতিরিক্ত খরচও অন্তর্ভুক্ত করে।
Note that the current national contract does not include any type of S sterilizer. Further items are added periodically and, therefore, it is important to check the contract for the latest information.
উল্লেখ্য যে বর্তমান জাতীয় চুক্তিতে কোনো প্রকার S জীবাণুমুক্তকরণ অন্তর্ভুক্ত নেই। আরও আইটেম পর্যায়ক্রমে যোগ করা হয় এবং তাই, সর্বশেষ তথ্যের জন্য চুক্তি পরীক্ষা করা গুরুত্বপূর্ণ।
All GDC registered dentists in Scotland can view the contract at the NHS National procurement website, CDSnet: www.scotcat.nhs.uk/cdsnet/cdsnet.asp (see Appendix 6 for further details.)
স্কটল্যান্ডের সমস্ত GDC নিবন্ধিত দাঁতের ডাক্তাররা NHS জাতীয় সংগ্রহের ওয়েবসাইট, CDSnet: www.scotcat.nhs.uk/cdsnet/cdsnet.asp-এ চুক্তিটি দেখতে পারেন (আরো বিশদ বিবরণের জন্য পরিশিষ্ট 6 দেখুন।)
Health Facilities Scotland and the chief Dental Officer recommend that all decontamination equipment (ultrasonic cleaners, washer disinfectors, and sterilizers) is purchased using the national contract as a guide. A sterilizer purchased via the contract will meet the specifications included in the points listed in Section. Provided that the additional installation commissioning, testing, and maintenance package is also purchased. The supplies are listed on CDSnet and need to be contacted directly to purchase equipment.
স্বাস্থ্য সুবিধা স্কটল্যান্ড এবং প্রধান ডেন্টাল অফিসার সুপারিশ করেন যে সমস্ত ডিকনট্যামিনেশন সরঞ্জাম (আল্ট্রাসনিক ক্লিনার, ওয়াশার ডিসইনফেক্টর এবং স্টেরিলাইজার) একটি গাইড হিসাবে জাতীয় চুক্তি ব্যবহার করে কেনা হয়। চুক্তির মাধ্যমে কেনা একটি জীবাণুনাশক অনুচ্ছেদে তালিকাভুক্ত পয়েন্টগুলিতে অন্তর্ভুক্ত বৈশিষ্ট্যগুলি পূরণ করবে৷ শর্ত থাকে যে অতিরিক্ত ইনস্টলেশন কমিশনিং, টেস্টিং এবং রক্ষণাবেক্ষণ প্যাকেজও কেনা হয়। সরবরাহগুলি CDSnet-এ তালিকাভুক্ত করা হয়েছে এবং সরঞ্জাম কেনার জন্য সরাসরি যোগাযোগ করতে হবে।
Consult the LDU equipment contract at www.scotcat.scot.nhs.uk/cdsnet/cdsnet.asp to inform purchasing decisions and consider quoting it when purchasing new equipment.
কেনার সিদ্ধান্ত জানাতে www.scotcat.scot.nhs.uk/cdsnet/cdsnet.asp-এ LDU সরঞ্জাম চুক্তির পরামর্শ নিন এবং নতুন সরঞ্জাম কেনার সময় এটি উদ্ধৃত করার কথা বিবেচনা করুন।
Failure to comply with the manufacturer’s instructions can adversely affect the safety of an instrument and affect product guarantees on warranties.
উত্পাদনের নির্দেশাবলী মেনে চলতে ব্যর্থতা একটি যন্ত্রের নিরাপত্তাকে বিরূপভাবে প্রভাবিত করতে পারে এবং ওয়ারেন্টিতে পণ্যের গ্যারান্টিকে প্রভাবিত করতে পারে
Check the manufacturer’s instructions before purchase to ensure that instruments are suitable, that is:
যন্ত্রগুলি উপযুক্ত কিনা তা নিশ্চিত করতে ক্রয়ের আগে নির্মাতাদের নির্দেশাবলী পরীক্ষা করে দেখুন, অর্থাৎ:
- they are good qualities and CE marked.
- তারা ভাল গুণাবলী এবং CE চিহ্নিত.
- they can withstand the temperature and pressure applied during the steam sterilization cycle used in your sterilization.
- তারা আপনার নির্বীজনে ব্যবহৃত বাষ্প নির্বীজন চক্রের সময় প্রয়োগ করা তাপমাত্রা এবং চাপ সহ্য করতে পারে।
- Whether there is a limit to how many times an instrument can be sterilized (e. g. electrosurgery tips).
- একটি যন্ত্র কতবার জীবাণুমুক্ত করা যায় তার একটি সীমা আছে কিনা (যেমন ইলেক্ট্রোসার্জারি টিপস)।
If there are reusable instruments in use that cannot withstand sterilization, source alternatives that can be sterilized or that are single-use.
যদি ব্যবহারে পুনঃব্যবহারযোগ্য যন্ত্র থাকে যা নির্বীজন প্রতিরোধ করতে পারে না, উৎস বিকল্প যা জীবাণুমুক্ত করা যেতে পারে বা যা একক ব্যবহার।
Staff Roles-
Appendix 4 details the personnel necessary for validation and quality assurance. This includes both staff in the practice and external personnel.
পরিশিষ্ট 4 বৈধতা এবং গুণমান নিশ্চিতকরণের জন্য প্রয়োজনীয় কর্মীদের বিবরণ। এর মধ্যে অনুশীলনের কর্মী এবং বহিরাগত কর্মী উভয়ই অন্তর্ভুক্ত।
Appoint a user as the named person responsible for appointing operators and ensuring their competence, and for the day to day management of each sterilize, its use, maintenance and testing and relevant documentation. In a dental practice, this role could be delegated to a suitably trained member of staff, for example, a senior dental nurse or practice manager.
অপারেটর নিয়োগ এবং তাদের দক্ষতা নিশ্চিত করার জন্য এবং প্রতিটি জীবাণুমুক্তকরণ, এর ব্যবহার, রক্ষণাবেক্ষণ এবং পরীক্ষা এবং প্রাসঙ্গিক ডকুমেন্টেশনের দৈনন্দিন ব্যবস্থাপনার জন্য দায়ী নামযুক্ত ব্যক্তি হিসাবে একজন ব্যবহারকারীকে নিয়োগ করুন। একটি ডেন্টাল অনুশীলনে, এই ভূমিকাটি উপযুক্তভাবে প্রশিক্ষিত কর্মীদের একজন সদস্যকে অর্পণ করা যেতে পারে, উদাহরণস্বরূপ, একজন সিনিয়র ডেন্টাল নার্স বা অনুশীলন ব্যবস্থাপক।
Appoint Operators to operate each sterilize, including performing basic housekeeping duties.
প্রতিটি জীবাণুমুক্ত করার জন্য অপারেটর নিয়োগ করুন, যার মধ্যে গৃহস্থালির মৌলিক দায়িত্ব পালন করা।
Staff Training for Sterilization-
It is a requirement of the provision and use of work Equipment Regulations 1998,Glennie Technical Requirements, HPS LDU Guidance and MDA DB 2002(06) that all staff manage, supervise or operate sterilizer are trained in their use and maintenance. The practice owner (Management refer to Appendix 4 for personnel) is responsible for ensuring that systems are in place for ongoing staff training.
কাজের সরঞ্জাম রেগুলেশন 1998, গ্লেনি টেকনিক্যাল রিকোয়ারমেন্টস, HPS LDU গাইডেন্স এবং MDA DB 2002(06) এর বিধান এবং ব্যবহারের জন্য এটি একটি প্রয়োজনীয়তা যে সমস্ত স্টাফ তাদের ব্যবহার এবং রক্ষণাবেক্ষণের বিষয়ে প্রশিক্ষিত হয়। অনুশীলনের মালিক (ব্যবস্থাপনা কর্মীদের জন্য পরিশিষ্ট 4 দেখুন) চলমান কর্মীদের প্রশিক্ষণের জন্য সিস্টেমগুলি রয়েছে তা নিশ্চিত করার জন্য দায়ী।
Ensure all members of the dental team who undertake decontamination of dental instruments are competent, supervised and trained. For sterilization the should-
নিশ্চিত করুন যে ডেন্টাল টিমের সকল সদস্য যারা ডেন্টাল ইন্সট্রুমেন্টগুলিকে দূষণমুক্ত করেন তারা দক্ষ, তত্ত্বাবধানে এবং প্রশিক্ষিত। জীবাণুমুক্ত করার জন্য উচিত-
- understand the procedures during the guidance.
- নির্দেশিকা চলাকালীন পদ্ধতিগুলি বুঝুন।
- know what kind of sterilizers are in the practice and what type of cycle is used in each sterilizer.
- অনুশীলনে কী ধরণের জীবাণুনাশক রয়েছে এবং প্রতিটি জীবাণুমুক্তকরণে কী ধরণের চক্র ব্যবহার করা হয় তা জানুন।
- know how to prepare the range of instruments used in practice correctly for sterilization, including new instruments, loading configuration, lubrication, inspection, wrapping, labelling.
- নতুন যন্ত্র, লোডিং কনফিগারেশন, তৈলাক্তকরণ, পরিদর্শন, মোড়ানো, লেবেলিং সহ জীবাণুমুক্তকরণের জন্য অনুশীলনে ব্যবহৃত যন্ত্রের পরিসর সঠিকভাবে কীভাবে প্রস্তুত করতে হয় তা জানুন।
- know how to store instruments after sterilization.
- জীবাণুমুক্ত করার পরে কীভাবে যন্ত্র সংরক্ষণ করতে হয় তা জানুন।
Refer to section of cleaning of dental instruments for general information about staff training, Hepatitis B vaccination and use of Personal Protective Equipment (PPE).
কর্মীদের প্রশিক্ষণ, হেপাটাইটিস বি টিকা এবং ব্যক্তিগত সুরক্ষামূলক সরঞ্জাম (পিপিই) ব্যবহার সম্পর্কে সাধারণ তথ্যের জন্য দাঁতের যন্ত্রপাতি পরিষ্কারের বিভাগটি পড়ুন।
Sterilization Workflow-
The decontamination process is carried out as a dirty-to-clean workflow within the Local Decontamination Unit (LDU). SHPN 13 Part 2 provides guidance on LDU design, including workflow.
লোকাল ডিকনট্যামিনেশন ইউনিট (LDU)-এর মধ্যে পরিচ্ছন্ন কর্মপ্রবাহ পরিষ্কার করার জন্য নোংরা হিসাবে বিশুদ্ধকরণ প্রক্রিয়া করা হয়। SHPN 13 পার্ট 2 কর্মপ্রবাহ সহ LDU ডিজাইনের নির্দেশিকা প্রদান করে।
After instrument cleaning, ensure the decontamination area has the following items for sterilization arranged in the order listed-
যন্ত্র পরিষ্কার করার পরে, নিশ্চিত করুন যে দূষণমুক্তকরণ এলাকায় তালিকাভুক্ত ক্রমানুসারে জীবাণুমুক্ত করার জন্য নিম্নলিখিত আইটেমগুলি রয়েছে-
- an area for loading unwrapped instruments into trays or cassettes for sterilization or for pre-sterilization wrapping or bagging instruments if using a vacuum sterilizer.
- একটি ভ্যাকুয়াম জীবাণুনাশক ব্যবহার করলে জীবাণুমুক্তকরণের জন্য বা প্রি-স্টেরিলাইজেশন র্যাপিং বা ব্যাগিং যন্ত্রের জন্য ট্রে বা ক্যাসেটে মোড়ানো যন্ত্র লোড করার একটি এলাকা।
- a steam sterilizer.
- একটি বাষ্প নির্বীজনকারী।
- an area for set down and cooling following removal from the sterilizer and for wrapping or bagging instruments that have been sterilized unwrapped.
- জীবাণুনাশক থেকে অপসারণের পরে সেট ডাউন এবং শীতল করার জন্য একটি এলাকা এবং মোড়ানো বা ব্যাগিং যন্ত্রের জন্য যেগুলিকে জীবাণুমুক্ত করা হয়েছে।
- a dedicated, clean, rigid, labelled box with a lid to transport instruments to the clinical or storage area safety and securely.
- ক্লিনিকাল বা স্টোরেজ এলাকায় নিরাপত্তা এবং নিরাপদে যন্ত্রপাতি পরিবহনের জন্য একটি ডেডিকেটেড, পরিষ্কার, অনমনীয়, ঢাকনাযুক্ত লেবেলযুক্ত বাক্স।
Ensure instrument storage is clean, orderly, enclosed (e. g. in trays, cassettes or pouches) and is not on open shelving.
নিশ্চিত করুন যে যন্ত্রের সঞ্চয়স্থান পরিষ্কার, সুশৃঙ্খল, আবদ্ধ (যেমন ট্রে, ক্যাসেট বা পাউচে) এবং খোলা তাক না।
- Ideally, instruments are stored in an area that is separate from the decontamination unit, well-lit, secure, dry and away from direct sunlight.
- আদর্শভাবে, যন্ত্রগুলি এমন একটি এলাকায় সংরক্ষণ করা হয় যা দূষণমুক্তকরণ ইউনিট থেকে পৃথক, ভালভাবে আলোকিত, নিরাপদ, শুষ্ক এবং সরাসরি সূর্যালোক থেকে দূরে।
Ensure storage is arranged so that sterile and sterilized instruments cannot be confused.
নিশ্চিত করুন যে স্টোরেজ ব্যবস্থা করা হয়েছে যাতে জীবাণুমুক্ত এবং জীবাণুমুক্ত যন্ত্রগুলি বিভ্রান্ত হতে না পারে।
3. Important Factors in Effective Sterilization-
3.1 Health & Safety Requirements for Small Steam Sterilizers
ছোট বাষ্প নির্বীজনকারীর জন্য স্বাস্থ্য ও নিরাপত্তার প্রয়োজনীয়তা-
The particular hazards associated with the use of steam sterilizers include burns from steam or hot metalwork (including instruments), explosive displacement of a door if not properly secured, and infection resulting from inadequate instrument processing. The Pressure System Safety Regulations 2000(PSSR) covers the installation and use of steam sterilizers. As a legal requirement, each sterilizer must have-
বাষ্প জীবাণুনাশক ব্যবহারের সাথে সম্পর্কিত বিশেষ বিপদগুলির মধ্যে রয়েছে বাষ্প বা গরম ধাতব কাজ (যন্ত্র সহ) থেকে পোড়া, সঠিকভাবে সুরক্ষিত না হলে দরজার বিস্ফোরক স্থানচ্যুতি এবং অপর্যাপ্ত যন্ত্র প্রক্রিয়াকরণের ফলে সংক্রমণ। প্রেসার সিস্টেম সেফটি রেগুলেশনস 2000(PSSR) স্টিম স্টেরিলাইজারের ইনস্টলেশন ও ব্যবহারকে কভার করে। একটি আইনি প্রয়োজন হিসাবে, প্রতিটি জীবাণুমুক্তকারীর অবশ্যই থাকতে হবে-
- a written scheme of examination.
- পরীক্ষার একটি লিখিত স্কিম।
- a periodic examination of the pressure system.
- চাপ সিস্টেমের একটি পর্যায়ক্রমিক পরীক্ষা।
- third-party liability insurance.
- তৃতীয় পক্ষের দায় বীমা।
- a record of repairs and maintenance of the pressure system.
- চাপ সিস্টেমের মেরামত এবং রক্ষণাবেক্ষণের একটি রেকর্ড।
Following installation and before use, obtain a written examination scheme for each sterilizer from the manufacturer, supplier, or insurer that has been prepared by a competent person (pressure vessels).
ইনস্টলেশনের পরে এবং ব্যবহারের আগে, প্রস্তুতকারক, সরবরাহকারী বা বীমাকারীর কাছ থেকে প্রতিটি জীবাণুনাশকের জন্য পরীক্ষার একটি লিখিত স্কিম নিন যা একজন দক্ষ ব্যক্তি (চাপ জাহাজ) দ্বারা প্রস্তুত করা হয়েছে।
Arrange for a competent person (pressure vessels) to conduct safety examinations in accordance with the written scheme of examination for the sterilizer, and retain a certificate as proof of each inspection. This examination is in addition to regular and routine maintenance.
জীবাণুনাশক পরীক্ষার লিখিত স্কিম অনুযায়ী নিরাপত্তা পরীক্ষা পরিচালনা করার জন্য একজন দক্ষ ব্যক্তির (চাপ জাহাজ) ব্যবস্থা করুন এবং প্রতিটি পরিদর্শনের প্রমাণ হিসাবে একটি শংসাপত্র রাখুন। এই পরীক্ষা নিয়মিত এবং নিয়মিত রক্ষণাবেক্ষণ ছাড়াও।
obtain third-party liability insurance that specifically covers risks associated with the operation of pressure vessels. Such as risks may not be covered by practice insurance.
তৃতীয় পক্ষের দায়বদ্ধতা বীমা প্রাপ্ত করুন যা বিশেষভাবে চাপবাহী জাহাজ পরিচালনার সাথে সম্পর্কিত ঝুঁকিগুলিকে কভার করে। যেমন ঝুঁকি অনুশীলন বীমা দ্বারা আচ্ছাদিত নাও হতে পারে.
To comply with legislation, keep records of all examinations and repairs to the pressure system.
আইন মেনে চলার জন্য, চাপ ব্যবস্থার সমস্ত পরীক্ষার এবং মেরামতের রেকর্ড রাখুন।
Your insurance company may provide details of competent persons (pressure vessels) or advice can be sought from an Authorising Engineer (Decontamination). The HSE leaflet Written schemes of examination provide further information. The competent person (pressure vessels) can also advise how frequently the safety examination is required for each sterilizer (typically at least once every 14 months).
আপনার বীমা কোম্পানী উপযুক্ত ব্যক্তিদের (চাপের জাহাজ) বিশদ বিবরণ দিতে পারে বা অনুমোদনকারী প্রকৌশলীর (ডিকনটামিনেশন) কাছ থেকে পরামর্শ চাওয়া যেতে পারে। পরীক্ষার HSE লিফলেট লিখিত স্কিম আরও তথ্য প্রদান করে। প্রতিটি জীবাণুনাশক (সাধারণত প্রতি 14 মাসে অন্তত একবার) জন্য কত ঘন ঘন নিরাপত্তা পরীক্ষা করা প্রয়োজন তাও উপযুক্ত ব্যক্তি (চাপবাহী জাহাজ) পরামর্শ দিতে পারেন।
3.2 Installation and Validation of Small Steam Sterilizers
ছোট বাষ্প নির্বীজন ইনস্টলেশন এবং বৈধতা-
To ensure that a small steam sterilizer reliably sterilizes each load, it is particularly important that the sterilizer is installed and commissioned correctly and that the sterilization process is validated for the specified load.
একটি ছোট বাষ্প নির্বীজনকারী নির্ভরযোগ্যভাবে প্রতিটি লোডকে জীবাণুমুক্ত করে তা নিশ্চিত করার জন্য, এটি বিশেষভাবে গুরুত্বপূর্ণ যে জীবাণুনাশকটি সঠিকভাবে ইনস্টল করা এবং চালু করা হয়েছে এবং নির্দিষ্ট লোডের জন্য নির্বীজন প্রক্রিয়াটি বৈধ করা হয়েছে।
Ensure that your supplier installs and commissions a new sterilizer and that a test personal (sterilizer) validates the sterilization process before use as specified in SHTM 2010 and MDA OB 2002 (06). Keep all records in the sterilizer logbook.
নিশ্চিত করুন যে আপনার সরবরাহকারী একটি নতুন জীবাণুনাশক ইনস্টল এবং কমিশন করে এবং SHTM 2010 এবং MDA OB 2002 (06) তে নির্দিষ্ট করা হিসাবে ব্যবহারের আগে একটি টেস্ট ব্যক্তিগত (স্ট্যারিলাইজার) জীবাণুমুক্তকরণ প্রক্রিয়াটিকে যাচাই করে৷ জীবাণুনাশক লগবুকে সমস্ত রেকর্ড রাখুন৷
3.3 Testing and Maintenance of Small Steam Sterilizers
ছোট বাষ্প নির্বীজন পরীক্ষা এবং রক্ষণাবেক্ষণ-
Ensure that each sterilizer 8s subject to a documented, planned maintenance program and periodic testing schedule, for example, through a service contract with your supplier or test person, or maintenance person.
নিশ্চিত করুন যে প্রতিটি জীবাণুনাশক 8গুলি একটি নথিভুক্ত, পরিকল্পিত রক্ষণাবেক্ষণ প্রোগ্রাম এবং পর্যায়ক্রমিক পরীক্ষার সময়সূচীর সাপেক্ষে, উদাহরণস্বরূপ, আপনার সরবরাহকারী বা পরীক্ষাকারী ব্যক্তি বা রক্ষণাবেক্ষণকারী ব্যক্তির সাথে একটি পরিষেবা চুক্তির মাধ্যমে৷
Record in the logbook details of all testing and maintenance carried out on each sterilizer.
প্রতিটি স্টেরিলাইজারে করা সমস্ত পরীক্ষা এবং রক্ষণাবেক্ষণের বিবরণ লগবুকে রেকর্ড করুন।
3.4 Cleanliness of Instruments’
যন্ত্রের পরিচ্ছন্নতা-
Contamination of instruments with residual tissue, body fluids, oil, or other deposits such as cement can prevent the direct contact between the steam and surfaces of the instruments that is necessary for effective sterilization. Also, any deposits left on instruments before sterilization might become fixed to the instruments making them more difficult to remove later. These deposits can also enter the water in the sterilizer reservoir and encourage the growth of microorganisms or accumulation of endotoxins, which could contaminate instruments processed subsequently.
অবশিষ্ট টিস্যু, শরীরের তরল, তেল বা অন্যান্য আমানত যেমন সিমেন্ট সহ যন্ত্রের দূষণ কার্যকর জীবাণুমুক্তকরণের জন্য প্রয়োজনীয় যন্ত্রগুলির বাষ্প এবং পৃষ্ঠের মধ্যে সরাসরি যোগাযোগ রোধ করতে পারে। এছাড়াও জীবাণুমুক্তকরণের আগে যন্ত্রগুলিতে থাকা যে কোনও আমানত যন্ত্রগুলিতে স্থির হয়ে যেতে পারে যা পরে অপসারণ করা আরও কঠিন করে তোলে। এই আমানতগুলি জীবাণুমুক্ত জলাধারের জলে প্রবেশ করতে পারে এবং অণুজীবের বৃদ্ধি বা এন্ডোটক্সিন জমা করতে উৎসাহিত করতে পারে, যা পরবর্তীতে প্রক্রিয়াকৃত যন্ত্রগুলিকে দূষিত করতে পারে। Ensure all items to be sterilized are clean and dry before placing them in the sterilizer chamber (see cleaning of dental instruments). জীবাণুমুক্ত করার জন্য সমস্ত আইটেমগুলি জীবাণুমুক্ত করার চেম্বারে রাখার আগে পরিষ্কার এবং শুকনো কিনা তা নিশ্চিত করুন (দন্তের যন্ত্রপাতি পরিষ্কার করা দেখুন)।
3.5 Loading of Instruments
Air removal might be impeded if instruments are not loaded correctly and steam may not contact every surface of every instrument. This steam contact is essential for sterilization to occur.
যন্ত্র লোড হচ্ছে যন্ত্রগুলি সঠিকভাবে লোড করা না হলে বায়ু অপসারণ বাধাগ্রস্ত হতে পারে এবং বাষ্প প্রতিটি যন্ত্রের প্রতিটি পৃষ্ঠের সাথে যোগাযোগ করতে পারে না। জীবাণুমুক্ত হওয়ার জন্য এই বাষ্পের যোগাযোগ অপরিহার্য।
- Load the sterilizer according to the manufacturers instructions and as specified at validation.
- নির্বীজনকারী লোড করুন প্রস্তুতকারকের নির্দেশাবলী অনুযায়ী এবং বৈধকরণের সময় নির্দিষ্ট করা।
- Ensure instruments do not overlap.
- যন্ত্রগুলি ওভারল্যাপ না হয় তা নিশ্চিত করুন।
- open hinged instruments to expose all of the surface area to the steam.
- বাষ্পের সাথে সমস্ত পৃষ্ঠের এলাকা উন্মুক্ত করার জন্য কব্জাযুক্ত যন্ত্রগুলি খুলুন।
- Place instruments on perforated trays, cassettes or racks that have been validated for use with the selected sterilization cycle.
- ছিদ্রযুক্ত ট্রে, ক্যাসেট বা র্যাকগুলিতে যন্ত্রগুলি রাখুন যা নির্বাচিত নির্বীজন চক্রের সাথে ব্যবহারের জন্য বৈধ করা হয়েছে।
- Do not overload the sterilizer chamber or individual trays or containers with instruments.
- জীবাণুনাশক চেম্বার বা পৃথক ট্রে বা যন্ত্র সহ পাত্রে ওভারলোড করবেন না।
3.6 Water for use in Steam Sterilizers-
Water used for sterilization must be essentially free of chemicals and endotoxins. In MDA DB2002(06), the MHRA recommends sterile water for irrigation BP though other forms of purified water of equivalent specification can be used, for example, certain freshly drawn reverse osmosis (RO) OR freshly prepared distilled waters. The use of tap water is not acceptable as this can lead to a build-up of contaminants that can be harmful and/or might damage the sterilizer.
জীবাণুমুক্ত করার জন্য ব্যবহৃত জল অবশ্যই রাসায়নিক এবং এন্ডোটক্সিন মুক্ত হতে হবে। MDA DB2002(06), MHRA সেচ BP-এর জন্য জীবাণুমুক্ত জলের সুপারিশ করে যদিও সমতুল্য স্পেসিফিকেশনের বিশুদ্ধ জলের অন্যান্য রূপ ব্যবহার করা যেতে পারে, উদাহরণস্বরূপ কিছু সতেজ টানা রিভার্স অসমোসিস (RO) বা সদ্য প্রস্তুত পাতিত জল। কলের জলের ব্যবহার গ্রহণযোগ্য নয় কারণ এর ফলে দূষিত পদার্থগুলি তৈরি হতে পারে যা ক্ষতিকারক হতে পারে এবং/অথবা জীবাণুনাশককে ক্ষতি করতে পারে।
- Fill the empty sterilizer reservoir with water of suitable quality. Do not use tap water.
- উপযুক্ত মানের জল দিয়ে খালি জীবাণুমুক্ত জলাধারটি পূরণ করুন। কলের জল ব্যবহার করবেন না।
- Change the water at least once per day or sooner if the chamber water is visibly coloured or cloudy. Record when each water change is done.
- চেম্বারের জল দৃশ্যমানভাবে রঙিন বা মেঘলা হলে দিনে অন্তত একবার বা তার আগে জল পরিবর্তন করুন। প্রতিটি জল পরিবর্তন করা হলে রেকর্ড করুন।
- If considering purchasing a water purification system to produce distilled or reverse osmosis water within the practice, first seek advice from an Authorising Engineer (Decontamination).
- অনুশীলনের মধ্যে পাতিত বা বিপরীত অসমোসিস জল উত্পাদন করার জন্য জল পরিশোধন ব্যবস্থা কেনার কথা বিবেচনা করলে, প্রথমে একজন অথরাইজিং ইঞ্জিনিয়ারের (ডিকনটামিনেশন) পরামর্শ নিন।
3.7 Sterilizer Logbook and Record Keeping-
A logbook is required for each sterilizer as a permanent record of the complete history of the sterilizer and could provide useful evidence in the event of an adverse incident. Alternative examples of pages of a sterilizer logbook are given in Appendix 7 and in MDA DB2002(06). Logbooks can also be purchased from Health Facilities Scotland.
জীবাণুনাশকের সম্পূর্ণ ইতিহাসের স্থায়ী রেকর্ড হিসাবে প্রতিটি জীবাণুনাশকের জন্য একটি লগবুক প্রয়োজন এবং প্রতিকূল ঘটনার ক্ষেত্রে দরকারী প্রমাণ সরবরাহ করতে পারে। জীবাণুনাশক লগবুকের পৃষ্ঠাগুলির বিকল্প উদাহরণগুলি পরিশিষ্ট 7 এবং MDA DB2002(06) এ দেওয়া হয়েছে৷ স্বাস্থ্য সুবিধা স্কটল্যান্ড থেকেও লগবুকগুলি কেনা যেতে পারে৷
Keep the logbook near the sterilizer so that routine information can be recorded easily.
জীবাণুনাশকের কাছে লগবুকটি রাখুন যাতে নিয়মিত তথ্য সহজেই রেকর্ড করা যায়।
Including in the logbook-
- installation, commissioning and validation tests and checks.
- ইনস্টলেশন, কমিশনিং এবং বৈধতা পরীক্ষা এবং চেক।
- the written scheme of examination under the pressure systems safety regulations 2000 (PSSR) (See Section3.1)
- প্রেসার সিস্টেম সেফটি রেগুলেশনস 2000 (PSSR) এর অধীনে পরীক্ষার লিখিত স্কিম (বিভাগ 3.1 দেখুন)
- a record of inspection under the scheme of examination.
- পরীক্ষার প্রকল্পের অধীনে পরিদর্শনের একটি রেকর্ড।
- results of periodic testing
- পর্যায়ক্রমিক পরীক্ষার ফলাফল
- a record of any cycle that fails and action taken, including what was done with the unsterilized load.
- যেকোন চক্রের একটি রেকর্ড যা ব্যর্থ হয় এবং গৃহীত পদক্ষেপ, যা নির্বীজিত লোড দিয়ে করা হয়েছিল,
- a record of all maintenance, repairs or medifications.
- সমস্ত রক্ষণাবেক্ষণ, মেরামত বা মেডিফিকেশনের রেকর্ড।
Retain the logbook for inspection.
পরিদর্শনের জন্য লগবুকটি রাখুন।
4. Sterilization Procedure
জীবাণুমুক্তকরণ পদ্ধতি-
The key consideration when determining sterilization operating procedures is the type of sterilizer and sterilization cycle that is being used because this dictates whether or not the instruments can be wrapped before sterilization if using a vacuum (or compatible Type S )sterilizer designed for wrapped instruments.
নির্বীজন অপারেটিং পদ্ধতি নির্ধারণ করার সময় মূল বিবেচ্য হল জীবাণুনাশক এবং জীবাণুমুক্তকরণ চক্রের ধরন যা ব্যবহার করা হচ্ছে কারণ এটি নির্ধারণ করে যে মোড়ানো যন্ত্রগুলির জন্য ডিজাইন করা ভ্যাকুয়াম (বা সামঞ্জস্যপূর্ণ টাইপ এস) জীবাণুনাশক ব্যবহার করলে নির্বীজন করার আগে যন্ত্রগুলিকে মোড়ানো যাবে কি না। Some procedures are common to all sterilizers and are described in 4.1 specific procedures for sterilizing unwrapped and wrapped instruments are then described in section 4.2 and 4.3 respectively. কিছু পদ্ধতি সব জীবাণুনাশকদের জন্য সাধারণ এবং 4.1 তে বর্ণনা করা হয়েছে 4.1 বিশেষ পদ্ধতিতে মোড়ানো এবং মোড়ানো যন্ত্রগুলিকে জীবাণুমুক্ত করার জন্য যথাক্রমে বিভাগ 4.2 এবং 4.3 এ বর্ণনা করা হয়েছে।
4.1 General Operation of Steam Sterilizers-
স্টিম স্টেরিলাইজারের সাধারণ অপারেশন-
Having in place a written sterilization procedure that is based on the manufacturer’s instructions, including loading, choice of sterilization cycle, procedure after sterilization, record keeping, and ensuring that all staff follow this written procedure.
একটি লিখিত নির্বীজন পদ্ধতি স্থাপন করা যা নির্মাতাদের নির্দেশাবলীর উপর ভিত্তি করে এবং এতে লোডিং, নির্বীজন চক্রের পছন্দ, জীবাণুমুক্তকরণের পরে পদ্ধতি এবং রেকর্ড রাখা এবং সমস্ত কর্মীরা এই লিখিত পদ্ধতি অনুসরণ করে তা নিশ্চিত করুন।
On each day that the sterilizer is used, carry out the daily housekeeping checks and daily tests.
জীবাণুনাশক ব্যবহার করা প্রতিটি দিনে, প্রতিদিনের হাউসকিপিং চেক এবং প্রতিদিনের পরীক্ষাগুলি সম্পাদন করুন
Ensure that maintenance and testing records for all sterilizers in use are up to date and satisfactory.
নিশ্চিত করুন যে ব্যবহার করা সমস্ত জীবাণুনাশকগুলির রক্ষণাবেক্ষণ এবং পরীক্ষার রেকর্ডগুলি আপ টু ডেট এবং সন্তোষজনক
4.1.1 Before Sterilization-
- Change you gloves and plastic apron before handling the cleaned instruments, remembering to wash your hands or use alcohol rub on visibly clean hands before putting on new gloves.
- পরিষ্কার করা যন্ত্রগুলি পরিচালনা করার আগে আপনার গ্লাভস এবং প্লাস্টিকের অ্যাপ্রোন পরিবর্তন করুন, আপনার হাত ধোয়ার কথা মনে রাখবেন বা নতুন গ্লাভস পরার আগে দৃশ্যমান পরিষ্কার হাতে অ্যালকোহল ঘষুন।
- If moving cleaned instruments to a sterilizer in another room , use a dedicated ,clean, rigid, labelled container with a lid.
- যদি পরিষ্কার করা যন্ত্রগুলিকে অন্য ঘরে জীবাণুমুক্ত করার যন্ত্রে স্থানান্তর করা হয় তবে একটি ঢাকনা সহ একটি উত্সর্গীকৃত, পরিষ্কার, কঠোর, লেবেলযুক্ত পাত্র ব্যবহার করুন
- Transfer instruments to the sterilizer as soon as possible after cleaning , thermal disinfection if a washer disinfector is used , drying and inspection for cleanliness and functionality. Only wrap instruments before sterilization if using vacuum sterilizer.
- পরিষ্কার করার পরে যত তাড়াতাড়ি সম্ভব জীবাণুমুক্ত করার যন্ত্রে স্থানান্তর করুন, যদি একটি ওয়াশার জীবাণুনাশক ব্যবহার করা হয় তবে তাপ জীবাণুনাশক, শুকানো এবং পরিচ্ছন্নতা এবং কার্যকারিতার জন্য পরিদর্শন। ভ্যাকুয়াম স্টেরিলাইজার ব্যবহার করলে শুধুমাত্র জীবাণুমুক্ত করার আগে যন্ত্রগুলি মোড়ানো।
- Load instruments correctly .For specific advice about unwrapped instruments refer to 4.2 and 4.3.
- যন্ত্রগুলিকে সঠিকভাবে লোড করুন। মোড়ানো যন্ত্র সম্পর্কে নির্দিষ্ট পরামর্শের জন্য 4.2 এবং 4.3 দেখুন।
- Check that there is sufficient water in the reservoir
- জলাধারে পর্যাপ্ত জল আছে কিনা তা পরীক্ষা করুন
- Select and start that sterilization cycle.
- নির্বাচন করুন এবং সেই নির্বীজন চক্র শুরু করুন।
- If instruments are not to be sterilized at the end of the day, clean and dry them, clearly label them as unsafe for handling or use, and reprocess them through the full decontamination cycle the next working day.
- যদি দিনের শেষে যন্ত্রগুলিকে জীবাণুমুক্ত করা না হয়, তবে সেগুলিকে পরিষ্কার এবং শুকিয়ে নিন, পরিষ্কারভাবে সেগুলি পরিচালনা বা ব্যবহারের জন্য অনিরাপদ হিসাবে লেবেল করুন এবং পরের কার্যদিবসে সম্পূর্ণ দূষণমুক্ত চক্রের মাধ্যমে পুনরায় প্রক্রিয়া করুন৷
4.1.2 After Sterilization-
Check the sterilizer to indicate that the cycle was satisfactory.
চক্রটি সন্তোষজনক ছিল তা নির্দেশ করতে জীবাণুমুক্তকরণ পরীক্ষা করুন।
Using the printout or data logger fitted to the sterilizer, confirm that the required temperature usually (134-137) was held for at least 3 minutes and if recorded, that the required pressure usually (2.1-2.25 bar)was attained during the cycle.
স্টেরিলাইজারে লাগানো প্রিন্টআউট বা ডেটা লগার ব্যবহার করে নিশ্চিত করুন যে প্রয়োজনীয় তাপমাত্রা সাধারণত (134-137) কমপক্ষে 3 মিনিট ধরে রাখা হয়েছিল এবং যদি রেকর্ড করা হয়, চক্রের সময় প্রয়োজনীয় চাপ সাধারণত (2.1-2.25 বার) অর্জিত হয়েছিল।
- In the absence of a printer or data logger that provides this information, manual monitoring and recording of each cycle is necessary. An Authorising Engineer (Decontamination) can advise on a suitable procedure. Upgrade to a machine with a suitable printer or data logger as soon as possible.
- এই তথ্য প্রদানকারী একটি প্রিন্টার বা ডেটা লগারের অনুপস্থিতিতে, প্রতিটি চক্রের ম্যানুয়াল পর্যবেক্ষণ এবং রেকর্ডিং প্রয়োজন। একজন অথরাইজিং ইঞ্জিনিয়ার (ডিকনটামিনেশন) একটি উপযুক্ত পদ্ধতি সম্পর্কে পরামর্শ দিতে পারেন। যত তাড়াতাড়ি সম্ভব একটি উপযুক্ত প্রিন্টার বা ডেটা লগার সহ একটি মেশিনে আপগ্রেড করুন।
Record that the cycle was satisfactory (e.g., sign the printout and retain it as a record).
রেকর্ড করুন যে চক্রটি সন্তোষজনক ছিল (যেমন প্রিন্টআউটে স্বাক্ষর করুন এবং এটিকে একটি রেকর্ড হিসাবে ধরে রাখুন)।
- Some practices choose to keep an electronic record by scanning signed printouts in batches, thus avoiding the need to store large quantities of printouts.
- কিছু অনুশীলন ব্যাচগুলিতে স্বাক্ষরিত প্রিন্টআউটগুলি স্ক্যান করে একটি ইলেকট্রনিক রেকর্ড রাখা বেছে নেয়, এইভাবে প্রচুর পরিমাণে প্রিন্টআউট সংরক্ষণ করার প্রয়োজন এড়ানো যায়।
Use special tray lifters or heatproof gloves to carefully unload the sterilizer.
সাবধানে জীবাণুমুক্ত করার জন্য বিশেষ ট্রে লিফটার বা হিটপ্রুফ গ্লাভস ব্যবহার করুন।
For instruments sterilized wrapped, check each package is satisfactory (as detailed in section 4.3.1)
নির্বীজিত মোড়ানো যন্ত্রগুলির জন্য, প্রতিটি প্যাকেজ সন্তোষজনক কিনা তা পরীক্ষা করুন (বিস্তারিত বিভাগ 4.3.1 এ)
If any of the above cycle conditions is not achieved or there is a problem with instruments unloaded from the sterilizer, ensure that the details are recorded, notify the user, and reprocess the instruments from the start of the decontamination cycle (cleaning, thermal disinfection if available, and sterilization).
যদি উপরোক্ত চক্রের শর্তগুলির মধ্যে কোনটি অর্জন না করা হয় বা জীবাণুনাশক থেকে আনলোড করা যন্ত্রগুলিতে সমস্যা হয় তবে নিশ্চিত করুন যে বিশদগুলি রেকর্ড করা হয়েছে, ব্যবহারকারীকে অবহিত করুন এবং দূষণমুক্তকরণ চক্রের শুরু থেকে যন্ত্রগুলি পুনরায় প্রক্রিয়া করুন (পরিষ্কার, তাপ নির্বীজন যদি উপলব্ধ, এবং নির্বীজন)।
4.1.3 At the end of the day
Follow the manufacturer’s instructions to drain and clean the chamber and reservoir at the end of each day and leave the day.
দিনের শেষে প্রতিটি দিন এবং ছুটির দিন শেষে চেম্বার এবং জলাশয় নিষ্কাশন এবং পরিষ্কার করার জন্য প্রস্তুতকারকের নির্দেশাবলী অনুসরণ করুন।
4.2 Unwrapped Instruments (All Sterilizers)
মোড়ানো যন্ত্র (সমস্ত নির্বীজনকারী)-
If using a non-vacuum sterilizer, the instruments must be processed unwrapped.
যদি একটি নন ভ্যাকুয়াম জীবাণুনাশক ব্যবহার করা হয়, তাহলে যন্ত্রগুলিকে আবৃত করে প্রক্রিয়া করতে হবে।
Solid instruments can be sterilized unwrapped in any type of sterilizer. The sterilization of hollow or lumened instruments can only be achieved if they are cleaned effectively and a vacuum (or a compatible Type S) sterilizer is used.
সলিড যন্ত্রগুলিকে যেকোন ধরণের জীবাণুমুক্ত করা যায় না। ফাঁপা বা লুমেনযুক্ত যন্ত্রগুলির জীবাণুমুক্তকরণ কেবল তখনই অর্জন করা যেতে পারে যদি সেগুলি কার্যকরভাবে পরিষ্কার করা হয় এবং একটি ভ্যাকুয়াম (বা একটি সামঞ্জস্যপূর্ণ টাইপ এস) জীবাণুমুক্তকরণ ব্যবহার করা হয়৷
When processing instruments using a non-vacuum sterilizer, ensure that the instruments are unwrapped.
একটি নন ভ্যাকুয়াম জীবাণুনাশক ব্যবহার করে যন্ত্র প্রক্রিয়াকরণ করার সময়, নিশ্চিত করুন যে যন্ত্রগুলি খোলা আছে।
- Note that the sterilization of the sterilization of the internal surfaces of instruments with lumens processed in a non-vacuum sterilizer cannot be guaranteed.
- নোট করুন যে একটি নন ভ্যাকুয়াম নির্বীজনকারীতে প্রক্রিয়াকৃত লুমেন সহ যন্ত্রগুলির অভ্যন্তরীণ পৃষ্ঠগুলির জীবাণুমুক্তকরণের গ্যারান্টি দেওয়া যায় না।
- Refer to section 1.4 regarding the sterilization of dental handpieces.
- দাঁতের হ্যান্ডপিস নির্বীজন সংক্রান্ত বিভাগ 1.4 পড়ুন।
If possible, process instruments using sterilization cycle with a drying stage.
যদি সম্ভব হয়, শুকানোর পর্যায় সহ জীবাণুমুক্তকরণ চক্র ব্যবহার করে যন্ত্রপাতি প্রক্রিয়া করুন।
When using a vacuum sterilizer, if the load includes hollow or lumened instruments, ensure that a drying stage is included.
ভ্যাকুয়াম স্টেরিলাইজার ব্যবহার করার সময়, যদি লোডের মধ্যে ফাঁপা বা লুমেনযুক্ত যন্ত্র থাকে, তাহলে নিশ্চিত করুন যে শুকানোর পর্যায় অন্তর্ভুক্ত রয়েছে।ভ্যাকুয়াম স্টেরিলাইজার ব্যবহার করার সময়, যদি লোডের মধ্যে ফাঁপা বা লুমেনযুক্ত যন্ত্র থাকে, তাহলে নিশ্চিত করুন যে শুকানোর পর্যায় অন্তর্ভুক্ত রয়েছে।
4.2.1 Handling and Storage of Unwrapped Instruments Immediately After Sterilization-
Sterilization (জীবাণুমুক্ত) করার পরপরই মোড়ানো যন্ত্রগুলির হ্যান্ডলিং এবং স্টোরেজ-
Instruments that have been sterilized unwrapped are designated as ‘sterilized only’. It is currently acceptable for instruments sterilized unwrapped to be kept for later use .However, they must be-
যে যন্ত্রগুলিকে র্যাপ করে Sterilization (জীবাণুমুক্ত) করা হয়েছে সেগুলিকে 'শুধু জীবাণুমুক্ত' হিসাবে মনোনীত করা হয়েছে। এটি বর্তমানে যন্ত্রানুষঙ্গের জন্য গ্রহণযোগ্য যা পরবর্তীতে ব্যবহারের জন্য রাখা হবে।
- dry-it is very important that instruments are completely dry when stored because dampness encourages growth of microorganisms and corrosion of instruments.
- শুষ্ক- এটি অত্যন্ত গুরুত্বপূর্ণ যে যন্ত্রগুলি সংরক্ষণ করার সময় সম্পূর্ণরূপে শুকিয়ে যায় কারণ স্যাঁতসেঁতে অণুজীবের বৃদ্ধি এবং যন্ত্রের ক্ষয়কে উৎসাহিত করে।
- Protected from contamination
- দূষণ থেকে সুরক্ষিত
- Stored correctly -note that storage of loose unwrapped instruments is unacceptable.
- সঠিকভাবে সংরক্ষণ করা হয়েছে – মনে রাখবেন যে আলগা মোড়ানো যন্ত্রের স্টোরেজ অগ্রহণযোগ্য।
Clean hands and put on clean gloves and a clean apron before handling unwrapped instruments that have been removed from the sterilizer. Take additional precautions if the instruments are still hot.
জীবাণুনাশক থেকে সরানো মোড়ানো যন্ত্রগুলি পরিচালনা করার আগে হাত পরিষ্কার করুন এবং পরিষ্কার গ্লাভস এবং একটি পরিষ্কার এপ্রোন পরুন। যন্ত্রগুলি এখনও গরম থাকলে অতিরিক্ত সতর্কতা অবলম্বন করুন।
Examine newly sterilized instruments visually for dryness. Ideally, the instruments will be dry on removal from the sterilizer but, if a drying cycle has not been used, manual drying using disposable, non-linting wipes may be necessary.
শুষ্কতার জন্য দৃশ্যত নতুন নির্বীজিত যন্ত্র পরীক্ষা করুন। আদর্শভাবে জীবাণুনাশক থেকে অপসারণের সময় যন্ত্রগুলি শুকিয়ে যাবে তবে, যদি শুকানোর চক্র ব্যবহার না করা হয় তবে ডিসপোজেবল, নন-লিন্টিং ওয়াইপ ব্যবহার করে ম্যানুয়াল শুকানোর প্রয়োজন হতে পারে।
Do not leave sterilized instruments exposed in the clinical environment.
জীবাণুমুক্ত যন্ত্রগুলি ক্লিনিকাল পরিবেশে উন্মুক্ত রাখবেন না।
Store instruments individually or in sets in clean, dry conditions and in a manner that prevents recontamination.
যন্ত্রগুলিকে পৃথকভাবে বা সেটে পরিষ্কার, শুষ্ক অবস্থায় এবং এমনভাবে সংরক্ষণ করুন যা পুনঃদূষণ প্রতিরোধ করে।
- Options include placing instruments in covered trays, cassettes, or clip-in-trays in enclosed boxes or cupboards in a rack system, or sealing within a clean, single-use, sterilization-grade wrapping material or self-seal sterilization bags/pouches.
- বিকল্পগুলির মধ্যে রয়েছে, ঢেকে রাখা ট্রে, ক্যাসেট বা ক্লিপ-ইন-ট্রে একটি র্যাক সিস্টেমে আবদ্ধ বাক্সে বা আলমারিতে যন্ত্র স্থাপন করা, বা পরিষ্কার, একক ব্যবহারের মধ্যে সিল করা, জীবাণুমুক্ত গ্রেডের মোড়ক উপাদান বা স্ব-সিল নির্বীজন ব্যাগ/পাউচ।
When labeling wrapped instruments write on the labels before attaching them to the wrapping directly with a ballpoint or felt pen as this might damage it.
মোড়ানো যন্ত্রগুলিকে লেবেল করার সময় বলপয়েন্ট বা অনুভূত কলম দিয়ে সরাসরি মোড়ানোর আগে লেবেলে লিখুন কারণ এটি ক্ষতি করতে পারে।
Store instruments in clean enclosed cupboards drawers or boxes in an orderly manner that avoids damaging the wrapping.
পরিষ্কার পরিচ্ছন্ন আলমারির ড্রয়ারে বা বাক্সে এমন সুশৃঙ্খলভাবে যন্ত্র সংরক্ষণ করুন যাতে মোড়ানোর ক্ষতি না হয়।
Do not store any instruments on open shelving or on work surfaces in clinical areas.
স্টোরেজের সময়কাল কমাতে একটি ফার্স্ট-ইন, ফার্স্ট-আউট স্টক রোটেশন ব্যবহার করুন।
Use a first-in, first-out stock rotation to minimize the duration of storage.
স্টোরেজের সময়কাল কমাতে একটি ফার্স্ট-ইন, ফার্স্ট-আউট স্টক রোটেশন ব্যবহার করুন।
4.3 Wrapped Instruments (Vacuum Sterilizers)
Instruments can only be processed wrapped in a vacuum (or a compatible type S) sterilizer that is designed for wrapped instruments. If using a non-vacuum sterilizer, refer to section 4.2.
যন্ত্রগুলিকে শুধুমাত্র একটি ভ্যাকুয়াম (বা একটি সামঞ্জস্যপূর্ণ টাইপ S) জীবাণুনাশক দিয়ে মোড়ানো প্রক্রিয়া করা যেতে পারে যা মোড়ানো যন্ত্রগুলির জন্য ডিজাইন করা হয়েছে। যদি একটি নন ভ্যাকুয়াম স্টেরিলাইজার ব্যবহার করেন, তাহলে বিভাগ 4.2 দেখুন।
As wrapping and labeling are part of the validation process, the sterilizer should be re-validated when introducing new wrapping and labeling.
যেহেতু মোড়ানো এবং লেবেলিং বৈধকরণ প্রক্রিয়ার অংশ, তাই নতুন মোড়ক এবং লেবেলিং প্রবর্তনের সময় জীবাণুমুক্তকরণকে পুনরায় যাচাই করা উচিত।
If wrapping instruments prior to sterilization in a vacuum sterilizer, ensure that
যদি ভ্যাকুয়াম স্টেরিলাইজারে Sterilization (জীবাণুমুক্ত) করার আগে যন্ত্রগুলি মোড়ানো হয়, তা নিশ্চিত করুন
- the wrapping material manufactures instructions are followed.
- মোড়ানো উপাদান নির্দেশাবলী অনুসরণ করা হয় উত্পাদন.
- the wrapping materials are compatible with the steam sterilization process (in dental practices, self -seal sterilization pouches are typically used.
- মোড়ানো উপকরণগুলি বাষ্প নির্বীজন প্রক্রিয়ার সাথে সামঞ্জস্যপূর্ণ (দন্তের অনুশীলনে, স্ব-সীল নির্বীজন পাউচগুলি সাধারণত ব্যবহৃত হয়।
- only a single layer of wrapping material is used.
- মোড়ানো উপাদান শুধুমাত্র একটি একক স্তর ব্যবহার করা হয়.
- each Instrument is wrapped separately or as a set of instruments for a single treatment held in a cassette that prevents them overlapping.
- প্রতিটি ইন্সট্রুমেন্ট আলাদাভাবে মোড়ানো হয় বা একটি ক্যাসেটে রাখা একক ট্রিটমেন্টের জন্য যন্ত্রের সেট হিসাবে যা তাদের ওভারল্যাপিং প্রতিরোধ করে।
- the correct size of pouch is used larger then the contents.
- থলির সঠিক আকার সামগ্রীর চেয়ে বড় ব্যবহার করা হয়।
- the method of sealing preserves the microbial barrier properties of the wrapping and enables the pack to be opened aseptically (self-seal or fold three times and apply autoclave tape).
- সিল করার পদ্ধতিটি মোড়ানোর মাইক্রোবিয়াল বাধা বৈশিষ্ট্যগুলিকে সংরক্ষণ করে এবং প্যাকটিকে অ্যাসেপ্টিকভাবে খোলার জন্য সক্ষম করে (সেলফ-সিল বা তিনবার ভাঁজ করুন এবং অটোক্লেভ টেপ প্রয়োগ করুন)।
Attach a pre-written or pre-printed adhesive label to each pack that includes the word ‘Sterile the process date, the sterilizer identification and cycle number. Do not write on the label after attaching it to the wrapping and do not write directly onto the wrapping with a ballpoint or felt pen as this might damage it
প্রতিটি প্যাকের সাথে একটি পূর্ব-লিখিত বা পূর্ব-মুদ্রিত আঠালো লেবেল সংযুক্ত করুন যাতে 'জীবাণুমুক্ত প্রক্রিয়ার তারিখ, জীবাণুনাশক সনাক্তকরণ এবং চক্র নম্বর' শব্দটি অন্তর্ভুক্ত থাকে। র্যাপিংয়ের সাথে সংযুক্ত করার পর লেবেলে লিখবেন না এবং বলপয়েন্ট বা অনুভূত কলম দিয়ে সরাসরি মোড়ানোর উপরে লিখবেন না কারণ এটি ক্ষতি করতে পারে।
Use chemical process indicator that is either printed on the pouch or available as a label or tape.
রাসায়নিক প্রক্রিয়া নির্দেশক ব্যবহার করুন যা হয় থলিতে মুদ্রিত বা লেবেল বা টেপ হিসাবে উপলব্ধ।
- Note that this does not indicate sterility but simply distinguishes items that have been exposed to a sterilization process from those that have not.
- মনে রাখবেন যে এটি বন্ধ্যাত্বের ইঙ্গিত দেয় না তবে কেবল এমন আইটেমগুলিকে আলাদা করে যা একটি জীবাণুমুক্তকরণ প্রক্রিয়ার সংস্পর্শে এসেছে যেগুলি নেই।
Ensure that the selected sterilization cycle includes a drying stage.
নিশ্চিত করুন যে নির্বাচিত নির্বীজন চক্র একটি শুকানোর পর্যায় অন্তর্ভুক্ত করে।
- It is essential to dry the load before the sterilizer chamber is opened otherwise the wrapped instruments will not remain sterile.
- জীবাণুনাশক চেম্বার খোলার আগে লোডটি শুকানো অপরিহার্য অন্যথায় মোড়ানো যন্ত্রগুলি জীবাণুমুক্ত থাকবে না।
4.3.1 Handling and Storage Wrapped Instruments Instruments Immediately After Sterilization.
Sterilization (জীবাণুমুক্ত) করণের পরপরই হ্যান্ডলিং এবং স্টোরেজ মোড়ানো যন্ত্রের যন্ত্র-
Careful handling and storage of sterilized packs will ensure that the contents remain sterilized until the pack is opened.
জীবাণুমুক্ত প্যাকগুলির যত্ন সহকারে পরিচালনা এবং স্টোরেজ নিশ্চিত করবে যে প্যাকটি খোলা না হওয়া পর্যন্ত সামগ্রীগুলি জীবাণুমুক্ত থাকবে।
Check the wrapping material for dampness tears, broken seals, or any other damage and that the label is intact and the details are legible.
স্যাঁতসেঁতে অশ্রু, ভাঙা সীল বা অন্য কোন ক্ষতির জন্য মোড়ানো উপাদান পরীক্ষা করুন এবং লেবেলটি অক্ষত আছে এবং বিশদটি পাঠযোগ্য।
It is very important that instrument that instruments are completely dry when stored because dampness encourages the growth of microorganisms and corrosion of instruments.
এটি অত্যন্ত গুরুত্বপূর্ণ যে যন্ত্রগুলি সংরক্ষণ করার সময় যন্ত্রগুলি সম্পূর্ণরূপে শুকিয়ে যায় কারণ স্যাঁতসেঁতে অণুজীবের বৃদ্ধি এবং যন্ত্রগুলির ক্ষয়কে উৎসাহিত করে৷
Handle packs carefully so that they are not dropped or damaged.
প্যাকগুলি সাবধানে হ্যান্ডেল করুন যাতে সেগুলি পড়ে না যায় বা ক্ষতিগ্রস্থ না হয়
Do not place newly sterilized wrapped instrument packs on cool or solid surfaces because these items are cooling fast and are in a vulnerable state because the warm vapor leaving the pack can condense to form dew that wets the wrapping materials.
নতুন জীবাণুমুক্ত মোড়ানো যন্ত্রের প্যাকগুলিকে শীতল বা শক্ত পৃষ্ঠে রাখবেন না কারণ এই আইটেমগুলি দ্রুত শীতল হয় এবং একটি অরক্ষিত অবস্থায় থাকে কারণ প্যাকটি ছেড়ে যাওয়া উষ্ণ বাষ্প ঘনীভূত হয়ে শিশির তৈরি করতে পারে যা মোড়ানোর উপকরণগুলিকে ভিজিয়ে দেয়।
If a wrapped item or pack is wet, is dropped on the floor, is torn, or has broken seals, it is no longer sterile. Unwrap the instruments and return them to the start of the decontamination process.
যদি একটি মোড়ানো আইটেম বা প্যাক ভিজে যায়, মেঝেতে ফেলে দেওয়া হয়, ছিঁড়ে যায় বা সিল ভেঙে যায় তবে এটি আর জীবাণুমুক্ত থাকে না। যন্ত্রগুলো খুলে ফেলুন এবং দূষণমুক্তকরণ প্রক্রিয়া শুরুতে ফিরিয়ে দিন।
If wrapped sterile instrument packs are to be stored for some time, confirm that the process date is marked clearly on the wrapping to enable stock rotation.
মোড়ানো জীবাণুমুক্ত ইন্সট্রুমেন্ট প্যাকগুলি যদি কিছু সময়ের জন্য সংরক্ষণ করা হয়, তবে নিশ্চিত করুন যে স্টক ঘূর্ণন সক্ষম করার জন্য প্রক্রিয়ার তারিখটি মোড়কে পরিষ্কারভাবে চিহ্নিত করা হয়েছে৷
Check that the chemical process indicator has changed color correctly. If it has not, investigate the problem, assess the disruption to the decontamination process, and reprocess the instruments from the start of the decontamination cycle.
রাসায়নিক প্রক্রিয়া নির্দেশক সঠিকভাবে রঙ পরিবর্তন হয়েছে তা পরীক্ষা করুন। যদি এটি না থাকে, সমস্যাটি তদন্ত করুন, দূষণমুক্ত প্রক্রিয়ার ব্যাঘাতের মূল্যায়ন করুন এবং দূষণমুক্ত চক্রের শুরু থেকে যন্ত্রগুলিকে পুনরায় প্রক্রিয়া করুন।
Store wrapped instruments in clean, enclosed cupboards, drawers, or boxes in an orderly manner that avoids damaging the wrapping (i.e. dry with little variation in temperature and minimal handling).
মোড়ানো যন্ত্রগুলিকে পরিষ্কার, আবদ্ধ আলমারি, ড্রয়ার বা বাক্সে সুশৃঙ্খলভাবে সংরক্ষণ করুন যাতে মোড়ানোর ক্ষতি না হয় (যেমন তাপমাত্রার সামান্য পরিবর্তন এবং ন্যূনতম পরিচালনার সাথে শুকনো)।
Do not store instruments on open shelving or on work surfaces in clinical areas.
ক্লিনিকাল এলাকায় খোলা তাক বা কাজের পৃষ্ঠে যন্ত্র সংরক্ষণ করবেন না।
Use a first-in, first-out stock rotation to minimize the duration of storage of sterile instruments.
জীবাণুমুক্ত যন্ত্রের সঞ্চয়ের সময়কাল কমাতে প্রথম ইন, ফার্স্ট-আউট স্টক রোটেশন ব্যবহার করুন।
5. Inspection of Instrument packs before use-
ব্যবহারের আগে উপকরণ প্যাক পরিদর্শন-
Ensure hands are clean and dry when handling instrument packs.
ইন্সট্রুমেন্ট প্যাকগুলি পরিচালনা করার সময় হাত পরিষ্কার এবং শুকনো নিশ্চিত করুন।
Check each pack is satisfactory before use. Do not use the instruments if either.
ব্যবহার করার আগে প্রতিটি প্যাক সন্তোষজনক কিনা তা পরীক্ষা করুন। যদি হয় যন্ত্র ব্যবহার করবেন না.
- the outer wrapping or seals are damaged.
- বাইরের মোড়ক বা সীল ক্ষতিগ্রস্ত হয়.
- the pack is moist (see photograph).
- প্যাকটি আর্দ্র (ছবি দেখুন)।
- the pack has labelling that is damaged or incorrect.
- প্যাকটিতে লেবেলিং আছে যা ক্ষতিগ্রস্ত বা ভুল।
- the pack has a process indicator that has not changed colour correctly or.
- প্যাকটিতে একটি প্রক্রিয়া নির্দেশক রয়েছে যা সঠিকভাবে রঙ পরিবর্তন করেনি বা।
- the instruments are visibly soiled.
- যন্ত্রগুলো দৃশ্যত নোংরা।
Instead, open the pack and return the instrument to the start of the decontamination process.
পরিবর্তে, প্যাকটি খুলুন এবং দূষণমুক্তকরণ প্রক্রিয়া শুরুতে যন্ত্রটিকে ফিরিয়ে দিন।
If an instrument appears damaged, remove it from use.
যদি কোন যন্ত্র ক্ষতিগ্রস্থ প্রদর্শিত হয়, এটি ব্যবহার থেকে সরান।
6. Validation, Periodic Testing, and Maintenance of small steam Sterilizers-
বৈধকরণ, পর্যায়ক্রমিক পরীক্ষা এবং ছোট বাষ্প নির্বীজন রক্ষণাবেক্ষণ- There is no practical way of determining that items processed in a steam sterilizer have been sterilized. Instead, tests need to be carried out regularly to confirm that during each sterilization cycle the sterilizer reproduces the operating conditions that were previously established as effective for sterilization. Essentially, testing is necessary to confirm that the machine reproducibly does what it was designed and set up to do. স্টিম স্টেরিলাইজারে প্রক্রিয়াকৃত আইটেমগুলি নির্বীজন করা হয়েছে তা নির্ধারণ করার কোন ব্যবহারিক উপায় নেই। পরিবর্তে, প্রতিটি নির্বীজন চক্রের সময় জীবাণু নির্বীজনকারী অপারেটিং অবস্থার পুনরুত্পাদন করে যা পূর্বে নির্বীজন করার জন্য কার্যকর হিসাবে প্রতিষ্ঠিত হয়েছিল তা নিশ্চিত করার জন্য নিয়মিত পরীক্ষা করা দরকার। মূলত, মেশিনটি পুনরুত্পাদনকারীভাবে যা ডিজাইন করা হয়েছিল এবং যা করার জন্য সেট আপ করা হয়েছিল তা নিশ্চিত করার জন্য পরীক্ষা করা প্রয়োজন।
Validation is a documented process used to show that sterilization will repeatedly and consistently take place to a satisfactory standard when defined operating conditions are used. These operating conditions include the choice of the sterilization cycle, the nature of the load, the loading pattern, wrapping, trays or containers, and labeling. Validation comprises a series of specified checks and tests carried out annually and as part of the commissioning process following the installation of a new sterilizer. These checks and tests are performed by Test Person (Sterilizers) as specified in SHTM 2010.
বৈধকরণ হল একটি নথি প্রক্রিয়া যা দেখানোর জন্য ব্যবহৃত হয় যে যখন সংজ্ঞায়িত অপারেটিং শর্তগুলি ব্যবহার করা হয় তখন নির্বীজন বারবার এবং ধারাবাহিকভাবে একটি সন্তোষজনক থেকে মান পর্যন্ত ঘটবে। এই অপারেটিং শর্তগুলির মধ্যে রয়েছে নির্বীজন চক্রের পছন্দ, লোডের প্রকৃতি, লোডিং প্যাটার্ন, মোড়ানো, ট্রে বা পাত্র এবং লেবেলিং। বৈধকরণের মধ্যে রয়েছে নির্দিষ্ট চেক এবং পরীক্ষাগুলির একটি সিরিজ যা বার্ষিক এবং একটি নতুন জীবাণুমুক্তকরণ ইনস্টল করার পরে কমিশনিং প্রক্রিয়ার অংশ হিসাবে করা হয়। এসএইচটিএম 2010-এ উল্লেখিত হিসাবে এই পরীক্ষাগুলি এবং পরীক্ষাগুলি টেস্ট পারসন (স্টেরাইলাইজার) দ্বারা সঞ্চালিত হয়।
In addition, satisfactory periodic testing is necessary to provide ongoing reassurance that the sterilizer is performing consistently as specified at validation. The legal requirement is to carry out periodic tests as specified in the sterilizer manufacturer’s instructions. Daily and weekly tests will normally be carried out by practice personnel and are described below. Quarterly (if specified by the manufacturer) and yearly (also known as annual revalidation) tests require specialist equipment and are performed by external personnel (test person sterilizers. If the manufacturer’s instructions are not available, periodic testing as recommended within SHTM 2010 is necessary.
উপরন্তু, সন্তুষ্টিজনক পর্যায়ক্রমিক পরীক্ষা করা আবশ্যক যে চলমান আশ্বাস প্রদান করে যে নির্বীজনকারীটি বৈধকরণের সময় নির্দিষ্ট করা অনুসারে ধারাবাহিকভাবে কাজ করছে। জীবাণুনাশক প্রস্তুতকারকদের নির্দেশাবলীতে উল্লেখিত পর্যায়ক্রমিক পরীক্ষা চালানোর জন্য আইনি প্রয়োজন। দৈনিক এবং সাপ্তাহিক পরীক্ষাগুলি সাধারণত অনুশীলন কর্মীদের দ্বারা পরিচালিত হবে এবং নীচে বর্ণনা করা হয়েছে। ত্রৈমাসিক (যদি প্রস্তুতকারকের দ্বারা নির্দিষ্ট করা হয়) এবং বার্ষিক (বার্ষিক পুনর্বিন্যাস হিসাবেও পরিচিত) পরীক্ষাগুলির জন্য বিশেষজ্ঞ সরঞ্জামের প্রয়োজন হয় এবং বহিরাগত কর্মীদের দ্বারা সঞ্চালিত হয় (একজন পরীক্ষার্থী নির্বীজনকারী৷ যদি নির্মাতার নির্দেশাবলী উপলব্ধ না হয়, SHTM 2010 এর মধ্যে সুপারিশকৃত পর্যায়ক্রমিক পরীক্ষা করা প্রয়োজন৷ .
Table 2 lists the periodic tests that SHTM 2010 part 3 and MDA DB2002(06) describe in detail for the various types of steam sterilizers. For specific guidance on testing and maintenance of type S sterilizers, refer to the Manufacturer’s instructions.
সারণি 2 পর্যায়ক্রমিক পরীক্ষার তালিকা দেয় যা SHTM 2010 অংশ 3 এবং MDA DB2002(06) বিভিন্ন ধরনের বাষ্প নির্বীজনকারীর জন্য বিশদভাবে বর্ণনা করে। টাইপ S জীবাণুমুক্তকরণের পরীক্ষা এবং রক্ষণাবেক্ষণের জন্য নির্দিষ্ট নির্দেশনার জন্য, নির্মাতাদের নির্দেশাবলী পড়ুন।
A planned program of preventive maintenance is also required for each sterilizer. Maintenance work is carried out by a qualified maintenance person. In some cases when parts (e.g. temperature probes) are changed, it is necessary to have the sterilization cycle revalidated.
প্রতিটি জীবাণুনাশকের জন্য প্রতিরোধমূলক রক্ষণাবেক্ষণের একটি পরিকল্পিত কর্মসূচিও প্রয়োজন। রক্ষণাবেক্ষণের কাজ একজন যোগ্যতাসম্পন্ন রক্ষণাবেক্ষণকারী ব্যক্তি দ্বারা পরিচালিত হয়। কিছু ক্ষেত্রে যখন অংশগুলি (উদাহরণস্বরূপ তাপমাত্রা অনুসন্ধান) পরিবর্তিত হয়, তখন জীবাণুমুক্তকরণ চক্রটি পুনরায় যাচাই করা প্রয়োজন।
For advice on validation of new or existing sterilizers, contact an Authorising Engineer (Decontamination)
নতুন বা বিদ্যমান জীবাণুমুক্তকরণের বৈধতা সম্পর্কে পরামর্শের জন্য, একজন অথরাইজিং ইঞ্জিনিয়ারের (ডিকনটামিনেশন) সাথে যোগাযোগ করুন। Obtain a written test schedule for each sterilizer from a test person (sterilizers) or an Authorising Engineer (Decontamination). একজন পরীক্ষক ব্যক্তি (জীবাণুমুক্তকারী) বা একজন অথরাইজিং ইঞ্জিনিয়ার (ডিকনটামিনেশন) এর কাছ থেকে প্রতিটি জীবাণুমুক্ত করার জন্য একটি লিখিত পরীক্ষার সময়সূচী পান।
6.1 Housekeeping Safety Checks
হাউসকিপিং সেফটি চেক-
6.1.1 Daily Housekeeping checks for all sterilizers
6.1.1 সমস্ত জীবাণুনাশকের জন্য দৈনিক হাউসকিপিং চেক
At the start of each day.
প্রতিটি দিনের শুরুতে।
Wipe the door seal with a clean, disposable, damp, non-linting cloth and carry out any other checks required by the manufacturer.
একটি পরিষ্কার, নিষ্পত্তিযোগ্য, স্যাঁতসেঁতে, নন-লিন্টিং কাপড় দিয়ে দরজার সিলটি মুছুন এবং প্রস্তুতকারকের দ্বারা প্রয়োজনীয় অন্য কোনও পরীক্ষা করুন৷
Check that the chamber and shelves are clean.
চেম্বার এবং তাক পরিষ্কার আছে কিনা দেখুন।
Refile the reservoir with suitable quality water (see section 3.6)
উপযুক্ত মানের জল দিয়ে জলাধার রিফিল করুন (বিভাগ 3.6 দেখুন)
When switching the power on, check that the ventilation louvers are not covered to avoid overheating.
পাওয়ার স্যুইচ করার সময়, অতিরিক্ত গরম এড়াতে বায়ুচলাচল ল্যুভার্স ঢেকে নেই তা পরীক্ষা করুন।
If recommended by the sterilizer manufacturer, preheat the sterilizer chamber before performing daily tests.
জীবাণুনাশক প্রস্তুতকারকের দ্বারা সুপারিশ করা হলে, প্রতিদিনের পরীক্ষা করার আগে জীবাণুমুক্ত করার চেম্বারটি প্রিহিট করুন।
Record the completion of the checks in the sterilizer logbook.
জীবাণুমুক্তকরণের লগবুকে চেকের সমাপ্তি রেকর্ড করুন।
6.1.2 Weekly Safety checks for all sterilizers-
6.1.2 সমস্ত জীবাণুমুক্ত করার জন্য সাপ্তাহিক নিরাপত্তা পরীক্ষা-
Before carrying out any weekly tests, the following checks are carried out in addition to the daily housekeeping checks.
কোনো সাপ্তাহিক পরীক্ষা করার আগে, প্রতিদিনের হাউসকিপিং চেক ছাড়াও নিম্নলিখিত চেকগুলি করা হয়।
- Examine the door seal for signs of wear damage.
- পরিধানের ক্ষতির লক্ষণগুলির জন্য দরজার সীলটি পরীক্ষা করুন।
- Examine the security and performance of the door safety features including the hinges and the locking mechanism as detailed in the manufacturers instructions.
- কব্জা এবং লকিং মেকানিজম সহ দরজা সুরক্ষা বৈশিষ্ট্যগুলির নিরাপত্তা এবং কার্যকারিতা পরীক্ষা করুন যেমন নির্মাতার নির্দেশাবলীতে বিস্তারিত রয়েছে।
- If a faullt is detected in the door seal or safety features ,ensure this is corrected before carrying out weekly tests or using the sterilizer.
- যদি দরজার সিল বা সুরক্ষা বৈশিষ্ট্যগুলিতে কোনও ত্রুটি ধরা পড়ে তবে সাপ্তাহিক পরীক্ষা বা জীবাণুমুক্ত করার আগে এটি সংশোধন করা হয়েছে তা নিশ্চিত করুন।
- Record satisfactory completion of the weekly safety checks in the sterilizer logbook.
- জীবাণুনাশক লগবুকে সাপ্তাহিক নিরাপত্তা পরীক্ষার সন্তোষজনক সমাপ্তি রেকর্ড করুন।
6.2 Automatic Control Test for All Sterilizers.
সমস্ত জীবাণুনাশক জন্য স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা.
The automatic controller is the device within the sterilizer that controls the sterilization cycle. To be sure that is working, an automatic control test is carried out every day either using the sterilization cycle parameter values recorded on the printout or electronic data logger or by manually observing and recording the cycle parameters if there is not a suitable recorded fitted. In addition, a manual automatic control test should also be carried out once per week for all sterilizers.
স্বয়ংক্রিয় নিয়ন্ত্রক হল জীবাণুনাশকের মধ্যে থাকা ডিভাইস যা জীবাণুমুক্তকরণ চক্রকে নিয়ন্ত্রণ করে। এটি কাজ করছে কিনা তা নিশ্চিত করার জন্য, প্রিন্টআউট বা ইলেকট্রনিক ডেটা লগারে রেকর্ড করা জীবাণুমুক্তকরণ চক্রের প্যারামিটার মান ব্যবহার করে, অথবা উপযুক্ত রেকর্ড করা না থাকলে সাইকেল প্যারামিটারগুলি ম্যানুয়ালি পর্যবেক্ষণ এবং রেকর্ড করার মাধ্যমে একটি স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা করা হয়। এছাড়াও, সমস্ত জীবাণুনাশকগুলির জন্য প্রতি সপ্তাহে একবার একটি ম্যানুয়াল স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা করা উচিত।
The automatic control test can be done when sterilizing a standard load unless also carrying out a steam penetration test for a vacuum sterilizer at the same time. This is usually the first cycle of the day.
একটি স্ট্যান্ডার্ড লোড নির্বীজন করার সময় স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা করা যেতে পারে, যদি না একই সময়ে ভ্যাকুয়াম নির্বীজনকারীর জন্য বাষ্প অনুপ্রবেশ পরীক্ষা করা হয়। এটি সাধারণত দিনের প্রথম চক্র।
6.2.1 Automatic Control Test Using a Recorded-
6.2.1 একটি রেকর্ড করা ব্যবহার করে স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা
This test is carried out once per day if the sterilizer is fitted with a suitable record (i.e. a printer or electronic data logger). If a suitable record is not fitted, a manual automatic control must be carried out each day (see section 6.2.2)
এই পরীক্ষাটি প্রতিদিন একবার করা হয় যদি জীবাণুনাশকটি উপযুক্ত রেকর্ড করা (যেমন একটি প্রিন্টার বা ইলেকট্রনিক ডেটা লগার) দিয়ে লাগানো থাকে। যদি একটি উপযুক্ত নথিবদ্ধ করা না থাকে, তাহলে প্রতিদিন একটি ম্যানুয়াল স্বয়ংক্রিয় নিয়ন্ত্রণ করা আবশ্যক (বিভাগ 6.2.2 দেখুন)
- Run a sterilization cycle with a standard load or an empty chamber (the chamber must be empty if a steam penetration test is also carried out in a vacuum sterilizer).
- একটি স্ট্যান্ডার্ড লোড বা একটি খালি চেম্বার সহ একটি জীবাণুমুক্ত করণ চক্র চালান (যদি ভ্যাকুয়াম নির্বীজনকারীতে বাষ্প প্রবেশের পরীক্ষা করা হয় তবে চেম্বারটি অবশ্যই খালি থাকতে হবে)।
- At the end of the cycle, check the printout or data logger to ensure that the recorded cycle parameters(temperature, pressure, hold me) are within the specified range for the cycle and comparable to the values obtained at
- চক্রের শেষে, প্রিন্টআউট বা ডেটা লগার পরীক্ষা করে নিশ্চিত করুন যে রেকর্ড করা চক্রের পরামিতিগুলি (তাপমাত্রা, চাপ, আমাকে ধরে রাখুন) চক্রের জন্য নির্দিষ্ট সীমার মধ্যে রয়েছে এবং প্রাপ্ত মানগুলির সাথে তুলনীয়
- Keep a record of the recorded values for temperature, pressure and hold time.
- তাপমাত্রা, চাপ এবং ধরে রাখার সময়ের জন্য রেকর্ড করা মানগুলির একটি রেকর্ড রাখুন।
- If the automatic control test is unsatisfactory (i.e. the recorded temperature, pressure or hold time are not within the specified range for the cycle) record the test as a fail and do not use the sterilizer until the fault has been resolved.
- যদি স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা অসন্তোষজনক হয় (অর্থাৎ রেকর্ড করা তাপমাত্রা, চাপ বা ধরে রাখার সময় চক্রের জন্য নির্দিষ্ট সীমার মধ্যে নয়) পরীক্ষাটিকে ব্যর্থ হিসাবে রেকর্ড করুন এবং ত্রুটিটি সমাধান না হওয়া পর্যন্ত জীবাণুনাশক ব্যবহার করবেন না।
- In this case, return any instruments that were loaded in the sterilizer to the start of the decontamination process.
- এই ক্ষেত্রে, জীবাণুমুক্তকরণ প্রক্রিয়ার শুরুতে জীবাণুমুক্তকরণে লোড করা যেকোন যন্ত্র ফিরিয়ে দিন।
- Sign the logbook.
- লগবুকে স্বাক্ষর করুন।
6.2.2 Manual Automatic Control Test
ম্যানুয়াল স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা
The test is carried out once per day if the sterilizer does not have a suitable recorded fitted, and once per week if there is a suitable recorder.
Run a sterilization cycle with a standard load or an empty chamber ( the chamber must be empty if also carrying out a steam penetration test in a vacuum sterilizer.
পরীক্ষাটি দিনে একবার করা হয় যদি জীবাণুনাশকটিতে উপযুক্ত রেকর্ড করা না থাকে, এবং যদি একটি উপযুক্ত রেকর্ডার থাকে তবে প্রতি সপ্তাহে একবার। একটি স্ট্যান্ডার্ড লোড বা একটি খালি চেম্বার সহ একটি জীবাণুমুক্তকরণ চক্র চালান ( যদি ভ্যাকুয়াম নির্বীজনকারীতে একটি বাষ্প অনুপ্রবেশ পরীক্ষা করা হয় তবে চেম্বারটি অবশ্যই খালি হতে হবে৷ জীবাণুনাশক যখন জীবাণুমুক্ত তাপমাত্রায় পৌঁছায় তখন চক্রের জীবাণুমুক্তকরণের সময়কাল শুরু করুন (এই পয়েন্টে পৌঁছানোর সময় প্রদর্শনটিও নির্দেশ করতে পারে)
Begin timing the sterilization hold period of the cycle when the sterilizer reaches the sterilizing temperature (the display might also indicate when this point is reached )
Note the bar pressure reached during the hold period
Note the temperature reached during the hold period.
জীবাণুনাশক যখন জীবাণুমুক্ত তাপমাত্রায় পৌঁছায় তখন চক্রের জীবাণুমুক্তকরণের সময়কাল শুরু করুন (এই পয়েন্টে পৌঁছানোর সময় প্রদর্শনটিও নির্দেশ করতে পারে) হোল্ড পিরিয়ডের সময় বারের চাপ পৌঁছেছে নোট করুন হোল্ড পিরিয়ডের সময় তাপমাত্রা পৌঁছেছে নোট করুন।
Record the temperature, pressure, and hold time in the sterilizer logbook, Record the test as a pass if these values are within the specified range for the cycle and comparable to the values obtained at validation.
If the automatic control test is unsatisfactory (i.e. the temperature, pressure, or hold time are not within the specified range for the cycle), record the test as a fail and do not use the sterilizer until the fault has been resolved.
Sign the logbook.
স্টেরিলাইজার লগবুকে তাপমাত্রা, চাপ এবং ধরে রাখার সময় রেকর্ড করুন, যদি এই মানগুলি চক্রের জন্য নির্দিষ্ট সীমার মধ্যে থাকে এবং বৈধকরণের সময় প্রাপ্ত মানগুলির সাথে তুলনীয় হয় তবে পরীক্ষাটিকে পাস হিসাবে রেকর্ড করুন। যদি স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা অসন্তোষজনক হয় (যেমন তাপমাত্রা, চাপ বা ধরে রাখার সময় চক্রের জন্য নির্দিষ্ট সীমার মধ্যে নয়), পরীক্ষাটিকে ব্যর্থ হিসাবে রেকর্ড করুন এবং ত্রুটিটি সমাধান না হওয়া পর্যন্ত জীবাণুমুক্তকরণ ব্যবহার করবেন না। লগবুকে স্বাক্ষর করুন।
6.3 Steam Penetration Test for Vacuum Sterilizers
ভ্যাকুয়াম স্টেরিলাইজারের জন্য স্টিম পেনিট্রেশন টেস্ট
This test is carried out in vacuum sterilizers designed to sterilize wrapped instruments. It is intended to show that steam will rapidly and evenly penetrate a test device that is similar to the intended load.
Refer to the sterilizer manufacturer’s instructions for the recommended test device and indicator.
এই পরীক্ষাটি ভ্যাকুয়াম স্টেরিলাইজারে করা হয় যা মোড়ানো যন্ত্রটিকে জীবাণুমুক্ত করার জন্য ডিজাইন করা হয়েছে। এটি দেখানোর উদ্দেশ্যে করা হয়েছে যে বাষ্প দ্রুত এবং সমানভাবে একটি পরীক্ষা ডিভাইসে প্রবেশ করবে যা উদ্দেশ্যযুক্ত লোডের অনুরূপ। প্রস্তাবিত পরীক্ষার ডিভাইস এবং নির্দেশকের জন্য জীবাণু নির্ণয়কারী নির্মাতাদের নির্দেশাবলী পড়ুন। দিনের শুরুতে, স্বাভাবিক জীবাণুমুক্তকরণ চক্রটি নির্বাচন করুন এবং পরীক্ষা ডিভাইস প্রস্তুতকারকদের নির্দেশাবলী অনুসরণ করে পরীক্ষা ডিভাইস ব্যতীত চেম্বার খালি রেখে পরীক্ষাটি করুন।
At the start of the day, select the usual sterilization cycle and perform the test with the chamber empty apart from the test device, following the test device manufacturer’s instructions.
Record whether the test was a pass or a fail in the sterilizer logbook.
If the steam penetration test result is unsatisfactory, repeat the test. A second unsatisfactory test result confirms that there is a fault. Arrange for a Maintenance person to investigate and do not use the sterilizer to sterilize instruments until the fault has been resolved.
দিনের শুরুতে, স্বাভাবিক জীবাণুমুক্তকরণ চক্রটি নির্বাচন করুন এবং পরীক্ষা ডিভাইস প্রস্তুতকারকদের নির্দেশাবলী অনুসরণ করে পরীক্ষা ডিভাইস ব্যতীত চেম্বার খালি রেখে পরীক্ষাটি করুন। স্টেরিলাইজার লগবুকে পরীক্ষাটি পাস বা ফেল ছিল কিনা তা রেকর্ড করুন। বাষ্প অনুপ্রবেশ পরীক্ষার ফলাফল অসন্তোষজনক হলে, পরীক্ষা পুনরাবৃত্তি করুন. একটি দ্বিতীয় অসন্তোষজনক পরীক্ষার ফলাফল নিশ্চিত করে যে একটি ত্রুটি আছে। তদন্ত করার জন্য একজন রক্ষণাবেক্ষণকারী ব্যক্তির ব্যবস্থা করুন এবং ত্রুটিটি সমাধান না হওয়া পর্যন্ত যন্ত্র জীবাণুমুক্ত করতে জীবাণুমুক্ত করার যন্ত্র ব্যবহার করবেন না।
6.4 Air leakage test for Vacuum Sterilizers
ভ্যাকুয়াম স্টেরিলাইজারের জন্য এয়ার লিকেজ পরীক্ষা
If air leaks into the sterilizer chamber at a higher rate than specified by the manufacturer. It might interfere with the penetration of steam into the load and as the air will not have passed through the bacteria retentive filter, there is a risk of recontaminating the load. An air leakage test involves removing air from the chamber, isolating the chamber, and monitoring the pressure for a period of time. Air leakage will cause and increase in the chamber pressure.
জীবাণুনাশক চেম্বারে বায়ু ফুটো হলে প্রস্তুতকারকের দ্বারা নির্দিষ্ট করা চেয়ে বেশি হারে। এটি লোডের মধ্যে বাষ্পের অনুপ্রবেশে হস্তক্ষেপ করতে পারে এবং যেহেতু বাতাস ব্যাকটেরিয়া রিটেনটিভ ফিল্টারের মধ্য দিয়ে যাবে না, তাই লোডটি পুনরায় দূষিত হওয়ার ঝুঁকি রয়েছে। একটি বায়ু ফুটো পরীক্ষা চেম্বার থেকে বায়ু অপসারণ, চেম্বার বিচ্ছিন্ন করা এবং একটি নির্দিষ্ট সময়ের জন্য চাপ নিরীক্ষণ জড়িত। বায়ু ফুটো হতে পারে এবং চেম্বার চাপ বৃদ্ধি হবে.
If an automatic test is available, carry out an air leakage test according to the manufacturer’s instructions once per week.
It is preferable to have a sterilizer that is capable of performing an automatic test because otherwise a test a person is required to perform a weekly manual test.
Note that some manufacturers specify that is carried out each day before the steam penetration test.
Record the results in the sterilizer logbook.
যদি একটি স্বয়ংক্রিয় পরীক্ষা উপলব্ধ হয়, প্রতি সপ্তাহে একবার প্রস্তুতকারকের নির্দেশাবলী অনুসারে একটি বায়ু ফুটো পরীক্ষা করুন। একটি স্বয়ংক্রিয় পরীক্ষা করতে সক্ষম এমন একটি জীবাণুনাশক থাকা বাঞ্ছনীয় কারণ অন্যথায় একটি পরীক্ষার জন্য একজন ব্যক্তির সাপ্তাহিক ম্যানুয়াল পরীক্ষা করতে হয়। নোট করুন যে কিছু নির্মাতারা নির্দিষ্ট করে যে বাষ্প অনুপ্রবেশ পরীক্ষার আগে প্রতিদিন বাহিত হয়। স্টেরিলাইজার লগবুকে ফলাফল রেকর্ড করুন।
6.5 Automatic Air Detection System Function Test for Vacuum Sterilizers
ভ্যাকুয়াম স্টেরিলাইজারের জন্য স্বয়ংক্রিয় এয়ার ডিটেকশন সিস্টেম ফাংশন টেস্ট
Sterilizers that actively remove air from the load before sterilization are fitted with a means of detecting whether any air present in the chamber is sufficient to impair sterilization during each cycle. A test is performed each week to check that the air detector is functioning correctly. The details of the test vary between different makes of the sterilizer. If the user cannot perform this test, it will require a test person to visit weekly to perform the test. This will add significantly to your costs so check this before purchase.
Carry out an automatic air detection system function test as specified in the manufacturer’s instructions once per week.
Record the results in the sterilizer logbook
জীবাণুমুক্ত করার আগে সক্রিয়ভাবে লোড থেকে বায়ু অপসারণকারী জীবাণুনাশকগুলি চেম্বারে উপস্থিত কোনো বায়ু প্রতিটি চক্রের সময় জীবাণুমুক্ত করার জন্য যথেষ্ট কিনা তা সনাক্ত করার একটি উপায়ে লাগানো হয়। এয়ার ডিটেক্টর সঠিকভাবে কাজ করছে কিনা তা পরীক্ষা করার জন্য প্রতি সপ্তাহে একটি পরীক্ষা করা হয়। নির্বীজনকারী বিভিন্ন তৈরির মধ্যে পরীক্ষার বিবরণ। যদি ব্যবহারকারী এই পরীক্ষাটি সম্পাদন করতে না পারে, তবে পরীক্ষাটি সম্পাদন করার জন্য একজন পরীক্ষার্থীকে সাপ্তাহিক পরিদর্শন করতে হবে। এটি আপনার খরচে উল্লেখযোগ্যভাবে যোগ করবে তাই কেনার আগে এটি পরীক্ষা করে দেখুন। প্রতি সপ্তাহে একবার মেইনফ্যাকচারারের নির্দেশাবলীতে উল্লেখিত হিসাবে একটি স্বয়ংক্রিয় বায়ু সনাক্তকরণ সিস্টেম ফাংশন পরীক্ষা করুন। স্টেরিলাইজার লগবুকে ফলাফল রেকর্ড করুন।
6.6 Other Periodic Tests
Arrange for a test person to carry out quarterly (if specified by the manufacturer) and yearly (annual revalidation) tests (e. g. through the purchase of a new sterilizer via the NHSScotland national contract with the full support package or by an arrangement with a contractor). A suitably experienced and qualified maintenance person may perform some of these tests (see appendix )
একজন পরীক্ষার্থীর জন্য ত্রৈমাসিক (যদি প্রস্তুতকারকের দ্বারা নির্দিষ্ট করা থাকে) এবং বার্ষিক (বার্ষিক পুনঃপ্রমাণ) পরীক্ষা করার ব্যবস্থা করুন (যেমন সম্পূর্ণ সমর্থন প্যাকেজ সহ NHSScotland জাতীয় চুক্তির মাধ্যমে বা একটি ঠিকাদারের সাথে একটি ব্যবস্থার মাধ্যমে একটি নতুন জীবাণুমুক্তকরণ কেনার মাধ্যমে)। একজন উপযুক্ত অভিজ্ঞ এবং যোগ্য রক্ষণাবেক্ষণকারী ব্যক্তি এই পরীক্ষাগুলির কিছু করতে পারেন (পরিশিষ্ট দেখুন)
6.7 Maintenance of Small Steam Sterilizers
ছোট বাষ্প নির্বীজন রক্ষণাবেক্ষণ
Obtain a maintenance contract to carry out the program of preventative maintenance tasks as specified in the manufacturer’s instructions.
If the manufacturer’s program of maintenance is not available, consult the Maintenance person (sterilizers) who might be an employee of the supplier or manufacturer ) to devise a suitable program.
Ensure details of all maintenance work are recorded in the sterilizer logbook, including problems, faults, and preventative and corrective actions.
If any maintenance or modification work is carried out to the pressure system, seek the advice of a competent person (pressure vessels) before using the sterilizer.
প্রস্তুতকারকের নির্দেশাবলীতে উল্লেখিত প্রতিরোধমূলক রক্ষণাবেক্ষণ কর্মের প্রোগ্রামটি সম্পাদন করার জন্য একটি রক্ষণাবেক্ষণ চুক্তি পান। যদি রক্ষণাবেক্ষণের জন্য প্রস্তুতকারকদের প্রোগ্রাম উপলব্ধ না হয়, একটি উপযুক্ত প্রোগ্রাম তৈরি করার জন্য রক্ষণাবেক্ষণকারী ব্যক্তির (জীবাণুমুক্তকারী) সাথে পরামর্শ করুন যিনি সরবরাহকারী বা প্রস্তুতকারকের একজন কর্মচারী হতে পারেন। সমস্ত রক্ষণাবেক্ষণ কাজের বিশদ সমস্যা, ত্রুটি, প্রতিরোধমূলক এবং সংশোধনমূলক পদক্ষেপ সহ জীবাণুমুক্তকরণের লগবুকে রেকর্ড করা হয়েছে তা নিশ্চিত করুন। যদি প্রেসার সিস্টেমে কোনো রক্ষণাবেক্ষণ বা পরিবর্তনের কাজ করা হয়, তাহলে জীবাণুনাশক ব্যবহার করার আগে একজন দক্ষ ব্যক্তির (চাপবাহী জাহাজ) পরামর্শ নিন।
Periodic tests for small steam sterilizers
ছোট বাষ্প নির্বীজন জন্য পর্যায়ক্রমিক পরীক্ষা
Daily Tests-
প্রতিদিনের পরীক্ষা-
- Automatic control test
- Steam penetration test
- স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা
- বাষ্প অনুপ্রবেশ পরীক্ষা
Weekly tests- (সাপ্তাহিক পরীক্ষা)-
Weekly safety checks (door seal and lock) Air leakage test (Automatic) Air detection system function test (automatic) Automatic control test
Steam Penetration test
- সাপ্তাহিক নিরাপত্তা পরীক্ষা (দরজা সিল এবং তালা)
- বায়ু ফুটো পরীক্ষা (স্বয়ংক্রিয়)
- বায়ু সনাক্তকরণ সিস্টেম ফাংশন পরীক্ষা (স্বয়ংক্রিয়)
- স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা
- বাষ্প অনুপ্রবেশ পরীক্ষা
Quarterly tests-
ত্রৈমাসিক পরীক্ষা-
- Weekly safety checks
- Air leakage test(automatic)
- Air leakage test (sensors connected)
- Automatic control test
- Verification of calibration of sterilizer instruments
- Thermometric test for a small load.
- Air leakage test (sensors removed).
- All detection system function test (automatic)
- Steam penetration test.
- সাপ্তাহিক নিরাপত্তা চেক
- বায়ু ফুটো পরীক্ষা (স্বয়ংক্রিয়)
- বায়ু ফুটো পরীক্ষা (সেন্সর সংযুক্ত)
- স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা
- জীবাণুমুক্ত যন্ত্রের ক্রমাঙ্কন যাচাইকরণ
- একটি ছোট লোড জন্য থার্মোমেট্রিক পরীক্ষা.
- বায়ু ফুটো পরীক্ষা (সেন্সর অপসারণ)।
- সমস্ত সনাক্তকরণ সিস্টেম ফাংশন পরীক্ষা (স্বয়ংক্রিয়)
- বাষ্প অনুপ্রবেশ পরীক্ষা.
Yearly and Revalidation tests-
বার্ষিক এবং পুনর্বিবেচনা পরীক্ষা-
- Yearly safety checks
- Steam non-condensable gas test
- Steam superheat test
- Steam dryness test
- Air leakage test (automatic)
- Air leakage test (sensors connected)
- Automatic control test.
- Verification of calibration of sterilizer instruments
- chamber overheat cut-out test.
- Air detector test for a small load.
- Air detector test for a full load
- Thermostatic test for a small load.
- Thermostatic test for a full load.
- Test for performance requalification as required by the user.
- Air leakage test (sensors removed)
- Air detection system function test (automatic)
- Steam penetration test.
The user may perform these tests only with the prior agreement by an Authorising Engineer (Decontamination)
বার্ষিক নিরাপত্তা পরীক্ষা
বাষ্প অ ঘনীভূত গ্যাস পরীক্ষা
বাষ্প সুপারহিট পরীক্ষা
বাষ্প শুষ্কতা পরীক্ষা
বায়ু ফুটো পরীক্ষা (স্বয়ংক্রিয়)
বায়ু ফুটো পরীক্ষা (সেন্সর সংযুক্ত)
স্বয়ংক্রিয় নিয়ন্ত্রণ পরীক্ষা।
জীবাণুমুক্ত যন্ত্রের ক্রমাঙ্কন যাচাইকরণ
চেম্বার ওভারহিট কাট-আউট পরীক্ষা।
একটি ছোট লোড জন্য এয়ার ডিটেক্টর পরীক্ষা.
একটি সম্পূর্ণ লোড জন্য এয়ার ডিটেক্টর পরীক্ষা
একটি ছোট লোড জন্য থার্মোস্ট্যাটিক পরীক্ষা.
একটি সম্পূর্ণ লোড জন্য থার্মোস্ট্যাটিক পরীক্ষা.
ব্যবহারকারীর প্রয়োজন অনুযায়ী কর্মক্ষমতা যোগ্যতার জন্য পরীক্ষা করুন।
বায়ু ফুটো পরীক্ষা (সেন্সর সরানো)
বায়ু সনাক্তকরণ সিস্টেম ফাংশন পরীক্ষা (স্বয়ংক্রিয়)
বাষ্প অনুপ্রবেশ পরীক্ষা.
ব্যবহারকারী এই পরীক্ষাগুলি শুধুমাত্র একজন অথরাইজিং ইঞ্জিনিয়ার (ডিকনট্যামিনেশন) এর পূর্বের চুক্তির মাধ্যমে করতে পারেন
 HRTD Medical Institute
HRTD Medical Institute